Abstract
Background
Left atrial–esophageal fistulas (LAEFs) are serious complications with high mortality after atrial fibrillation radiofrequency ablation (AFRA). Decreasing the incidence of esophageal thermal lesions (EsoTLs) that may lead to LAEFs is important. The aim of this study was to suppress EsoTL development and determine the appropriate alarm setting for a temperature-monitoring probe by using steerable sheath (STS) methods.
Methods
We enrolled 82 consecutive patients (mean, 61.9±11.7 years; 75.6% men) who underwent AFRA, including pulmonary vein isolation for symptomatic, drug-refractory atrial fibrillation with esophageal temperature monitoring by using STS between January 2011 and April 2014. All patients underwent upper gastrointestinal endoscopy (UGE) 1–3 days after AFRA. The timing of ablation discontinuation in the first 17 patients was determined by each physician during AFRA (only monitoring group, OM). In the next 65 patients, physicians were to immediately discontinue ablation when an alarm set at 39 °C went off (instruction group, INS). We compared two groups with respect to the incidence of EsoTLs.
Results
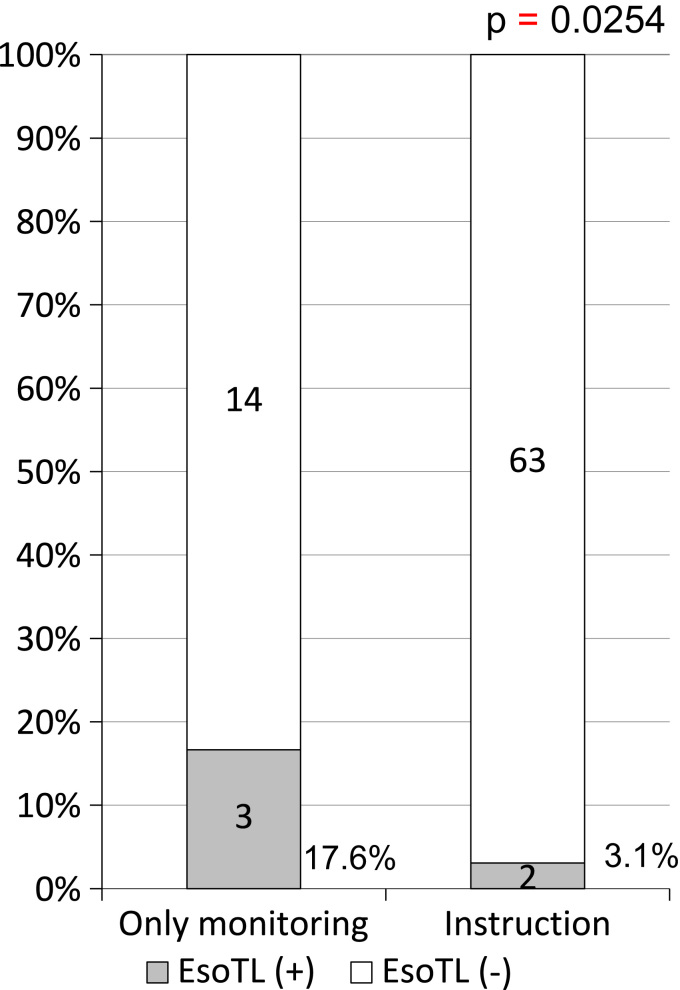
Among the 82 patients, 5 (6.1%) had EsoTLs after AFRA. EsoTLs occurred in 3 of 17 patients (17.6%) and 2 of 65 patients (3.1%) in the OM and INS groups, respectively. The incidence of EsoTLs in the INS group was significantly lower than that in the OM group (p=0.0254). EsoTL did not occur at maximal temperature less than 39 °C, measured by using esophageal temperature-monitoring probe.
Conclusions
Immediate discontinuation of ablation during pulmonary vein isolation remarkably decreased the incidence of EsoTLs, even when using STS.
Keywords: Atrial fibrillation ablation, Esophageal monitoring, Esophageal thermal lesion, Steerable sheath
1. Introduction
Atrial fibrillation radiofrequency ablation (AFRA) is useful for controlling the rhythm of atrial fibrillation (AF) [1], [2], [3]. However, severe complications rarely occur. Particularly, left atrial–esophageal fistulas (LAEF) caused by ablation energy is a fatal complication because the left atrial wall is adjacent to the esophagus. Patients with LAEF has been reported to have a high mortality [4]. Therefore, preventing esophageal thermal lesions (EsoTLs) caused by AFRA is important, and esophageal temperature monitoring during AFRA has been reported to effectively prevent LAEF [5], [6]. A steerable sheath (STS) is gradually brought into widespread use because AF recurrence rate after AFRA was lower when using STS than that when using non-STS [7]. However, the appropriate setting for esophageal temperature monitoring during pulmonary vein isolation remains unclear when STS. The aim of this study was to examine the association between the incidence of EsoTLs and the endoluminal temperature of the esophagus during AFRA, to identify the appropriate temperature for the esophageal temperature-monitoring probe, and how to decrease the incidence of EsoTLs when using the STS.
2. Materials and methods
2.1. Study population
A total of 82 consecutive patients (mean 61.9±11.7 years; 75.6% men) who underwent AFRA, included pulmonary vein isolation for symptomatic, drug-refractory AF with esophageal temperature monitoring between January 2011 and April 2014 were enrolled in the study. All patients underwent upper gastrointestinal endoscopy (UGE) several days after AFRA and had oral anticoagulation therapy for more than four weeks before AFRA.
This study conformed to the institutional guidelines of Showa University System, and the protocol was approved by the institutional review board of Showa University. All patients provided written informed consent.
2.2. Electrophysiology study
2.2.1. Atrial fibrillation radiofrequency ablation and temperature monitoring
Intravascular introducer sheaths were placed in the left subclavian vein, both femoral veins, and left femoral artery. A duo-decapolar catheter was positioned in the right atrium and coronary sinus. A decapolar catheter was positioned in the right ventricle. All patients were studied under dexmedetomidine (α-agonist) sedation with/without thiopental.
The NAVX-Ensite system (St. Jude Medical, Inc., St Paul, MN, USA) or The Carto3 system (Biosense Webster, Inc., Diamond Bar, CA, USA) was used for non-fluoroscopic, three-dimensional catheter orientation, and computed tomographic image integration. All patients were inserted with a temperature probe (Sensitherm, St. Jude Medical, Inc.) with five electrodes (three intermediate electrodes with a thermocouple) into the esophagus at the level of the left atrium (LA).
The esophageal temperature probe was visualized by three-dimensional mapping systems. After one transseptal puncture and two long sheath insertion in the LA, ablation and ring catheters were positioned, and the catheter ablation procedure was performed by using STS (Agilis, St. Jude Medical, Inc.).
Our AFRA procedure was essentially ipsilateral pulmonary vein isolation. Line ablations (mitral isthmus, roof, and bottom lines in the LA) were added in patients with persistent AF. An irrigated catheter was used with an initial maximum power of 25–35 W, depending on each physician and by the dragging method with STS, and the duration of one ablation was limited to less than 10 s on the posterior wall of the LA. Electrical isolation of all PVs was attempted with bidirectional conduction block in all patients.
Antiarrhythmic drugs (Na channel blockers, bepiridil, and amiodarone) were discontinued before AFRA. After AFRA, all patients received oral anticoagulation drugs again at the same day of AFRA and started proton-pump inhibitor therapy for four weeks,
2.2.2. Alarm setting of the esophageal temperature-monitoring probe
The alarm setting of the esophageal temperature-monitoring probe was set at 39 °C in all patients. All procedures in the first 17 patients were performed with STS between January 2011 and September 2012, and the timing of ablation discontinuation was determined by each physician during AFRA (only monitoring group, OM), which means that the temperature targets for ablation discontinuation were dependent on each physician. All procedures in the next 65 patients were performed with STS between October 2012 and April 2014. All physicians were instructed to discontinue ablation immediately when the alarm set at 39 °C was triggered (instruction group, INS), even if the ablation had been performed for only several seconds at one time. Re-ablation at the same site was performed with lower power than that used for the previous ablation repeatedly and intermittently after temperature normalized (less than 37.5 °C). One person immediately checked the maximum temperature when the alarm set at 39 °C was triggered.
2.2.3. Upper gastrointestinal endoscopy
Endoscopy was performed 1–3 days after the ablation procedure in all patients who underwent AFRA to detect EsoTLs. Lidocaine spray and midazolam for local anesthesia and conscious sedation, respectively, were used. The esophagus, stomach, and duodenum were observed. An endoscopy was performed again after several weeks if the EsoTLs were detected by the first endoscopy to make sure EsoTLs healed.
2.3. Statistical analysis
Continuous variables with normal distributions are summarized using means±standard deviation. Between-group differences were evaluated by using analysis for variance for continuous variables, Fisher׳s exact test for categorical variables, and univariate and multivariate logistic analyses with commercially available software (JMP SAS 11.0; SAS Institute Inc., Cary, NC).
3. Results
3.1. Patient characteristics
The patient characteristics in the OM and INS groups are shown in Table 1. A total of 82 patients were enrolled in the study retrospectively and assigned to the OM (n=17) or the INS group (n=65). The mean age of the patients in both groups (60.7±13.5 years and 62.0±11.6 years in OM and INS, respectively) was similar. No significant difference in the individual components of sex, AF type, LA diameter measured by using ultrasoundcardiography, body mass index, and the distance between the LA and esophagus measured by using computed tomography (CT).
Table 1.
Patient characteristics.
| Only monitoring | Instruction | p Value | |
|---|---|---|---|
| Number | 17 | 65 | |
| Age (years) | 60.7±13.5 | 62.0±11.6 | 0.72 |
| Sex (male) | 11 (64.7%) | 51 (78.5%) | 0.58 |
| Type of AF (paroxysmal) | 4 (76.5%) | 35 (53.9%) | 0.09 |
| Hypertension | 7 (41.2%) | 36 (55.4%) | 0.27 |
| Coronary artery disease | 1 (1.2%) | 2 (2.7%) | 0.61 |
| Congestive heart failure | 2 (11.7%) | 11 (11.6%) | 0.57 |
| LA diameter (mm) | 42.5±6.6 | 44.6±6.3 | 0.23 |
| Ejection fraction (%) | 59.6±8.3 | 56.0±12.5 | 0.16 |
| CHADS2 score (IQR) | 1.0 (0–1.0) | 1.2 (0–2) | 0.78 |
| BMI | 23.7±3.4 | 24.3±3.4 | 0.47 |
| LA-Eso (mm) | 1.70±0.38 | 1.76±0.45 | 0.54 |
AF, atrial fibrillation; BMI, body mass index; LA, left atrium;
LA-Eso, distance between the left atrium and esophagus.
3.2. Esophageal thermal lesions
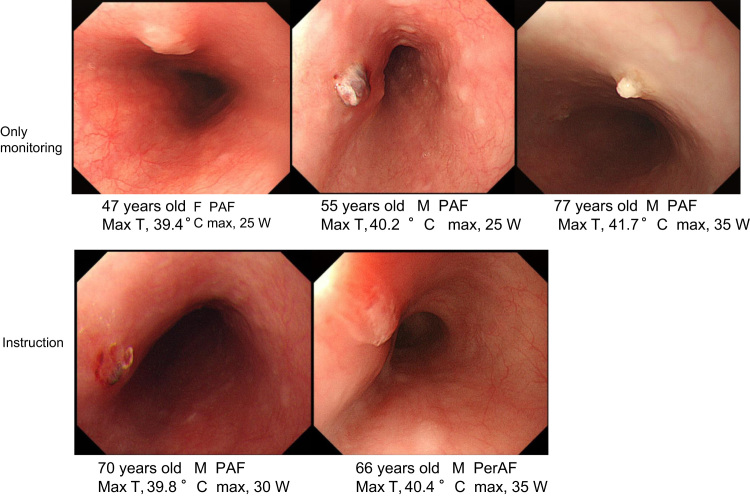
Of the 82 patients, 5 (6.1%) had EsoTLs after AFRA, wherein 3 of 17 patients (17.6%) and 2 of 65 patients (3.1%) in the OM and INS groups, respectively, had EsoTLs; the EsoTL incidence was significantly lower in the INS group (p=0.0254) (Fig. 1). The rate of changing the ablation line in the posterior wall was 0 of 17 patients (0%) and 4 of 65 patients (6.2%) in the OM and INS groups, respectively. Maximum ablation power at the posterior wall of the LA in patients with EsoTLs was 25 W, 25 W, and 35 W in the OM group and 30 W and 35 W in the INS group (Fig. 2). EsoTL was not observed at the maximum ablation power of 25 W or less in the INS group. One patient in the INS group had gastroparesis at the maximum ablation power of 35 W. No patients developed LAEF.
Fig. 1.
Incidence of esophageal thermal lesions.
Fig. 2.
Endoscopic findings of esophageal thermal lesions.
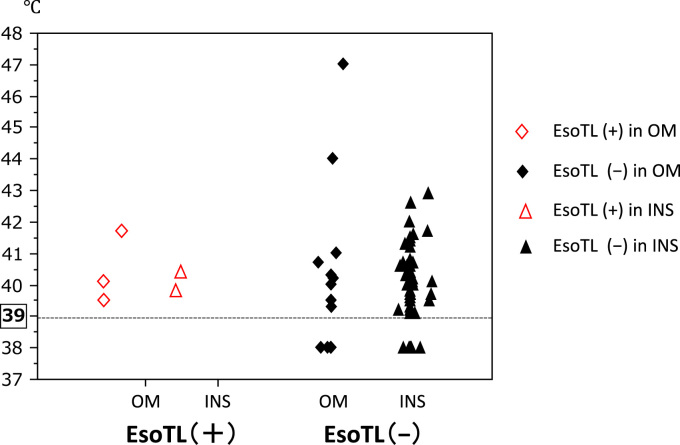
Fig. 3 shows the relationship of the maximum temperature of esophageal temperature-monitoring probe and development of EsoTLs in all patients. The dots were located at 38.0 °C in cases wherein the temperature alarm was not triggered at 39.0 °C, which meant that the temperature was lower than 39.0 °C. EsoTL did not occur with esophageal temperatures lower than 39.0 °C during ablation. Two patients in OM group and no patient in INS group had a maximum temperature higher than 43 °C. However, they had no EsoTL. All EsoTLs caused by AFRA was confirmed to be treated by esophageal endoscopy approximately one month with proton-pump inhibitor therapy alone.
Fig. 3.
Esophageal thermal lesions and maximum esophageal temperatures. Temperatures lower 39 °C are marked at 38 °C.
3.3. Follow up
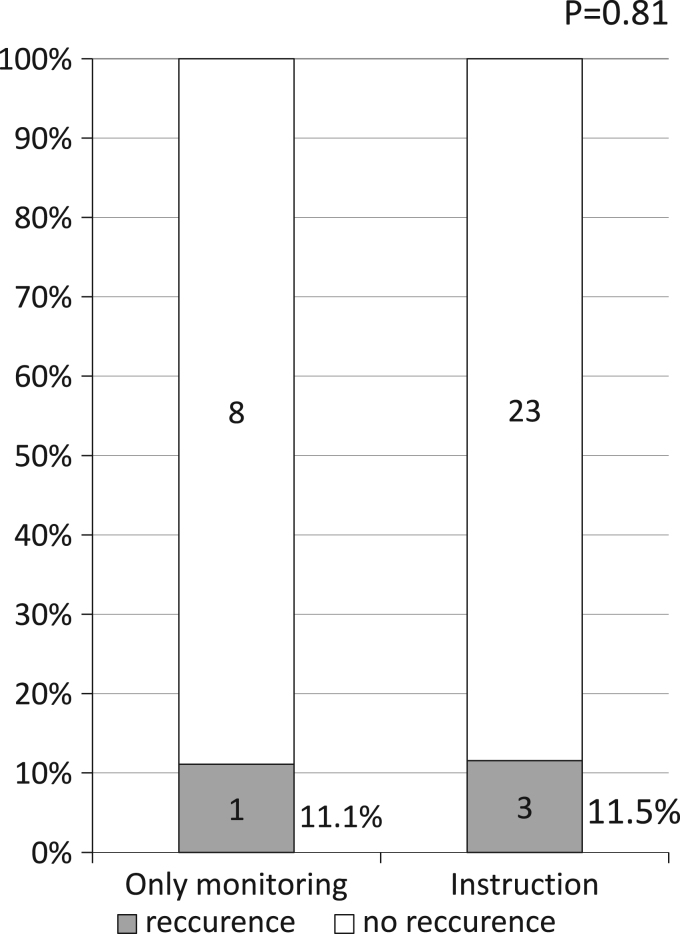
Fig. 4 showed AF recurrence rate in paroxysmal AF patients undergoing first pulmonary vein isolation. AF recurrence rate was 11.1% and 11.5% at the mean follow-up period of 682 days and 463.1 days, respectively, in the OM and INS groups, respectively. No significant difference was found in the AF recurrence rate between the OM and INS groups (p=0.81).
Fig. 4.
Recurrence rate in patients paroxysmal AF undergoing first pulmonary vein isolation.
3.3.1. Prediction of the development of esophageal thermal lesions
In univariate and multivariate analyses, the incidence of EsoTLs after AFRA was not significantly associated with any factors (age, sex, AF type [paroxysmal], hypertension, LA diameter, ejection fraction, CHADS2 score, body mass index, distance between the LA and esophagus, maximum temperature, and number of alarms) (Table 2).
Table 2.
Prediction of esophageal thermal lesions.
|
Univariate analysis | |
|---|---|
| Variables | p Value |
| Age | 0.904 |
| Gender (male) | 0.387 |
| Type of AF (paroxysmal) | 0.389 |
| Hypertension | 0.597 |
| LA diameter (mm) | 0.432 |
| Ejection fraction (% ) | 0.504 |
| CHADS2 score | 0.288 |
| BMI | 0.865 |
| LA-Eso (mm) | 0.421 |
| Maximum temperature | 0.823 |
| Number of alarm | 0.580 |
AF, atrial fibrillation; BMI, body mass index; LA, left atrium;
LA-Eso, distance between the left atrium and esophagus.
4. Discussion
4.1. Our experience with esophageal lesions
The OM group had one EsoTL following ablation discontinuation with an alarm setting of 41 °C esophageal temperature, with ablation energy of 25 W using STS, and EsoTL did not occur when using non-STS (0 of 13 patients) in the OM group. Subsequently, we started to immediately discontinue ablation an alarm setting of 39 °C esophageal temperature, and EsoTL did not occur in the INS group with ablation using 25 W or less even when using STS.
Little is known on the relationship between the development of EsoTLs and immediately discontinuing ablation when using STS.
The important findings of this study are the following. (1) The incidence of EsoTLs was remarkably decreased even when using STS by instructing the physicians to discontinue ablation immediately when the alarm of the esophageal temperature-monitoring probe, which was set at 39 °C, is triggered. (2) EsoTL did not occur with maximal esophageal temperatures lower than 39 °C. (3) EsoTL did not occur with maximal ablation power of 25 W or less in INS group.
4.2. Non-steerable and steerable sheaths
Ullah et al. reported that the contact force was greater with STS than with non-STS [7]. The mean total contact force was significantly higher in the STS group (16.9±4.0 g) than in the non-STS group (12.9±4.9 g). Especially in the posterior wall, a more significant difference in the contact force was found between the STS and non-STS groups. This implies that better contact is achieved by using STS than by using non-STS, but the incidence of EsoTLs is more likely to be higher with STS than with non-STS.
However, Piorkowski et al. reported that the AF recurrence rate and procedure time for AFRA were significantly lower with STS than with non-STS [7]. The same finding was seen at our institute; wherein the AF recurrence rate after AFRA was lower with STS than with a non-STS (6.7% vs. 16.4%; P<0.05) during a mean follow-up period of two years (unpublished data). This explains why we aggressively use STS.
4.3. Incidence of esophageal thermal lesions
We decided to choose an esophageal temperature setting of 39 °C, including safety margin because one EsoTL occurred in OM group at the maximum temperature even at 39.4 °C. The incidence of EsoTLs after AFRA has been reported under esophageal temperature monitoring. The incidence of EsoTLs varies [5], [6], [8], [9], [10], [11]. The incidence of EsoTLs in previous studies was 0–20% and 14.6–27% when using non-STS using STS (Table 2). However, we demonstrated that immediate ablation discontinuation when temperature reached 39 °C decreased the incidence of EsoTLs even with STS (17.1% in the OM group vs. 3.1% in the INS group, p=0.0254), Furthermore, EsoTL was not found at a power limit of 25 W or less in the INS group in the present study.
4.4. Alarm setting of esophageal temperature monitoring and ablation power
The results of the present and previous studies are shown in Table 3. Halm et al. reported that EsoTLs should be expected if the esophageal temperature reaches 41 °C or higher during the ablation procedure [6]. In the present study, a maximal temperature of 41 °C or higher was not related to an increased incidence of EsoTLs. However, if the esophageal temperature did not reach a temperature of 39 °C, EsoTL was not found. Although both, the study of Fernand et al. and the present study used STS and an alarm setting of 39 °C, the incidence of EsoTLs was different in the two studies [5].They reported that the duration of one ablation was limited to 20–30 s when creating lesions in the posterior LA. In the present study, duration of one ablation was limited to less than 10 s. This difference in duration of one ablation may have affected the incidence of EsoTLs between two studies.
Table 3.
Incidence of esophageal injury in the present and previous studies.
| Number of patients | Power limit | Measured temperature | Esophageal observation | Incidence of EsoTLs | |
|---|---|---|---|---|---|
| Steerable sheath | |||||
| Present study (OM) | 17 | 25–35 W | 39 °C | UGE | 17.6% |
| Present study (INS) | 65 | 25–35 W | 39 °C | UGE | 3.1% |
| Contreras-Valdes et al. [5] | 219 | 25 W | 39 °C | UGE | 27.0% |
| Halm et al. [6] | 185 | 30–40 W | 41 °C | UGE | 14.6% |
| Non-steerable sheath | |||||
| Leite et al. [8] | 45 | 25 W | 2 °C above baseline | UGE | 0% |
| Singh et al. [9] | 81 | 35 W | 38 °C | UGE | 6% |
| Kuwahara et al. [10] | 50 | 25–35 W | 42 °C | UGE | 20% |
| Sause et al. [11] | 184 | 30 W | 40 °C | UGE | 1.6% |
EsoTL, esophageal thermal lesion; INS, immediate discontinuation of the ablation group; OM, only the monitoring group; UGE, upper gastrointestinal endoscopy.
4.5. Predictors of esophageal thermal lesions
Several factors have been associated with the development of EsoTLs, such as pericardial fat and BMI [12], [13]. Pericardial fat between the esophagus and LA was present in only 10% of patients, all of whom were overweight, and none of the patients at below-normal weight had pericardial fat pads in the study. No pericardial fat pads between the esophagus and LA, as defined in the previous study, were found in the present study. The pericardial fat pad volume is reported to be positively correlated to the BMI [13]. The mean BMI (24±3.4 kg/m2) of the present study was lower than that of a previous study [14]. Therefore, we were not able to detect excessive pericardial fat pads in this study. The BMI was reported as one independent predictor for EsoTLs in Japanese patients without using esophageal thermal probes. However, in the present study, BMI and other factors (such as age, sex, AF type, hypertension, LA diameter, ejection fraction, CHADS2 score, the distance between LA and esophagus, maximum esophageal temperature, and number of alarm) were not related to the incidence of EsoTLs.
It is important to pay special attention to the development of EsoTLs in patients with a lower BMI, such as Asians. However, immediate ablation discontinuation under esophageal temperature monitoring has the potential to decrease the incidence of EsoTLs.
4.6. Limitations
This study had several limitations. First, it was not a prospective, randomized, multicenter study, which allows the presence of confounding variables interfering with the analysis. Second, ablation power when ablating the posterior LA was dependent on each physician, and the ablation power settings varied. Third, the measurement system of the esophageal thermal probe does not allow data storage. Recording temperature curves over time was not possible; therefore, the association of EsoTLs with rapid temperature increase and the duration of time with a temperature >39 °C were not evaluated. Further, large, multicenter studies are needed to determine the adequate esophageal temperature setting to prevent EsoTLs.
5. Conclusions
Immediate ablation discontinuation during pulmonary vein isolation remarkably decreased the incidence of EsoTLs, even when using STS. EsoTL did occur after AFRA with immediate ablation discontinuation at the esophageal temperature probe alarm setting of 39 °C, with an ablation power of 25 W, even when using STS.
Funding sources
None.
Conflict of interest
All authors declare no conflict of interest related to this study.
Acknowledgments
The authors would like to express their gratitude to all members of the Gastroenterology Department in our university for their contribution to this study.
References
- 1.Camm A.J., Kirchhof P., Lip G.Y. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12:1360–1420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 2.Camm A.J., Lip G.Y., De Caterina R. focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;2012(33):2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 3.Jais P., Cauchemez B., Macle L. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–2505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 4.Pappone C., Oral H., Santinelli V. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004;109:2724–2726. doi: 10.1161/01.CIR.0000131866.44650.46. [DOI] [PubMed] [Google Scholar]
- 5.Contreras-Valdes F.M., Heist E.K., Danik S.B. Severity of esophageal injury predicts time to healing after radiofrequency catheter ablation for atrial fibrillation. Heart Rhythm. 2011;8:1862–1868. doi: 10.1016/j.hrthm.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Halm U., Gaspar T., Zachaus M. Thermal esophageal lesions after radiofrequency catheter ablation of left atrial arrhythmias. Am J Gastroenterol. 2009;105:551–556. doi: 10.1038/ajg.2009.625. [DOI] [PubMed] [Google Scholar]
- 7.Piorkowski C., Eitel C., Rolf S. Steerable versus nonsteerable sheath technology in atrial fibrillation ablation: a prospective, randomized study. Circ Arrhythm Electrophysiol. 2011;4:157–165. doi: 10.1161/CIRCEP.110.957761. [DOI] [PubMed] [Google Scholar]
- 8.Leite L.R., Santos S.N., Maia H. Luminal esophageal temperature monitoring with a deflectable esophageal temperature probe and intracardiac echocardiography may reduce esophageal injury during atrial fibrillation ablation procedures: results of a pilot study. Circ Arrhythm Electrophysiol. 2011;4:149–156. doi: 10.1161/CIRCEP.110.960328. [DOI] [PubMed] [Google Scholar]
- 9.Singh S.M., d׳Avila A., Doshi S.K. Esophageal injury and temperature monitoring during atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2008;1:162–168. doi: 10.1161/CIRCEP.107.789552. [DOI] [PubMed] [Google Scholar]
- 10.Kuwahara T., Takahashi A., Takahashi Y. Incidences of esophageal injury during esophageal temperature monitoring: a comparative study of a multi-thermocouple temperature probe and a deflectable temperature probe in atrial fibrillation ablation. J Interv Card Electrophysiol. 2014;39:251–257. doi: 10.1007/s10840-013-9868-5. [DOI] [PubMed] [Google Scholar]
- 11.Sause A., Tutdibi O., Pomsel K. Limiting esophageal temperature in radiofrequency ablation of left atrial tachyarrhythmias results in low incidence of thermal esophageal lesions. BMC Cardiovasc Disord. 2010;10:52. doi: 10.1186/1471-2261-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamasaki H., Tada H., Sekiguchi Y. Prevalence and characteristics of asymptomatic excessive transmural injury after radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm. 2011;8:826–832. doi: 10.1016/j.hrthm.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 13.Lemola K., Sneider M., Desjardins B. Computed tomographic analysis of the anatomy of the left atrium and the esophagus: implications for left atrial catheter ablation. Circulation. 2004;110:3655–3660. doi: 10.1161/01.CIR.0000149714.31471.FD. [DOI] [PubMed] [Google Scholar]
- 14.Rosito G.A., Massaro J.M., Hoffmann U. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]