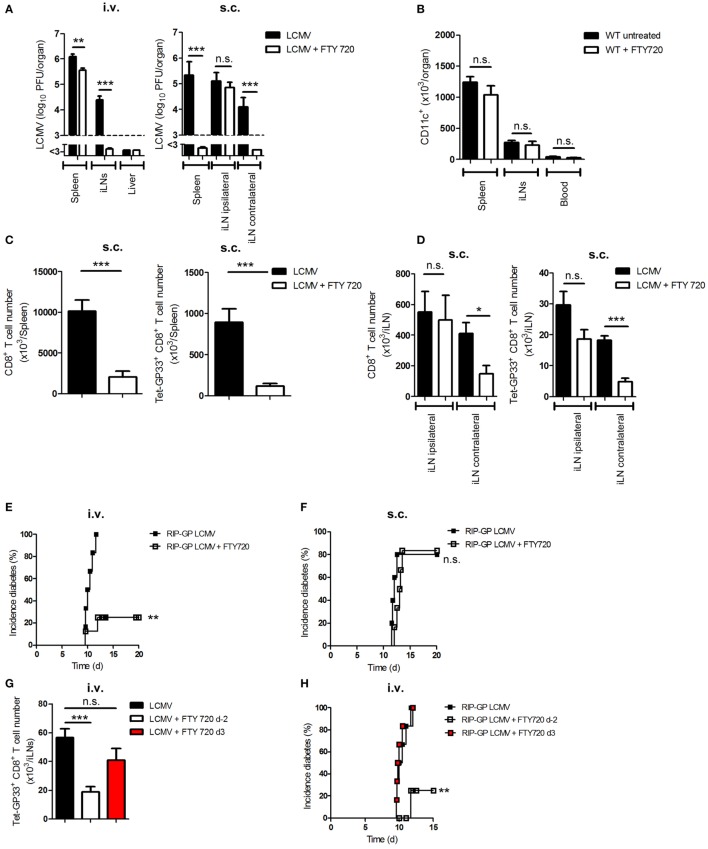
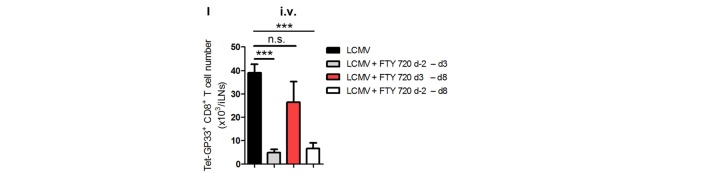
Figure 5.
FTY720 prevents viral transfer to lymph nodes and thereby inhibits the onset of diabetes. (A) C57BL/6 wild-type (WT) mice were treated daily with a sphingosine-1-phosphate antagonist [FTY720; 1 mg/kg body weight (bw)] starting from day −2 or were left untreated. Mice were infected intravenously (i.v.) or subcutaneously (s.c.) with 20 plaque-forming units (PFUs) of lymphocytic choriomeningitis virus strain WE (LCMV-WE). Viral titer was measured in the spleen, inguinal lymph nodes (iLNs) and liver on day 3 (d3; i.v.) or day 6 (d6; s.c.) after infection (n = 6; pooled from two experiments). Black column = LCMV; white column = LCMV + FTY720. (B) C57BL/6 WT mice were treated daily with FTY720 (1 mg/kg bw) starting from day −2 or were left untreated. CD11c+ cells in the spleen, iLNs, and blood were counted on day 0 (n = 6, pooled from two experiments). Black column = WT untreated; white column = WT + FTY720. (C,D) C57BL/6 WT mice were treated daily with FTY720 (1 mg/kg bw) starting on day −2 or were left untreated. Mice were infected s.c. with 20 PFU LCMV-WE. On day 12, total and LCMV-specific CD8+ T cells were counted in the spleen (C) and iLNs (D) (n = 6, pooled from two experiments). Black column = LCMV; white column = LCMV + FTY720. (E,F) Transgenic mice expressing the lymphocytic choriomeningitis virus (LCMV) glycoprotein (GP) under control of the rat insulin promoter (RIP-GP) were treated daily with FTY720 (1 mg/kg bw) starting from day −2 or were left untreated. Mice were intravenously or subcutaneously infected with 20 PFU LCMV-WE. Onset of diabetes was measured (E) i.v., n = 6–8, pooled from two experiments; (F) s.c., n = 5–6, pooled from two experiments. Black squares = RIP-GP LCMV; white squares = RIP-GP LCMV + FTY720. (G) C57BL/6 WT mice were treated daily with a sphingosine-1-phosphate antagonist [FTY720; 1 mg/kg body weight (bw)] starting from day −2 or 3 of infection or left untreated. Mice were infected intravenously (i.v.) with 20 PFU of LCMV-WE. On day 12, tetramer (Tet) GP33-specific CD8+ T cells in iLNs were counted by flow cytometry (n = 8, pooled from two experiments). Black column = LCMV; white column = LCMV + FTY720 (starting day −2); red column = LCMV + FTY720 (starting d3). (H) Transgenic mice expressing the LCMV glycoprotein (GP) under control of the rat insulin promoter (RIP-GP) were treated daily with FTY720 (1 mg/kg bw) starting from day −2 or 3 of infection or were left untreated. Mice were intravenously infected with 20 PFU LCMV-WE. Onset of diabetes was measured (n = 6, pooled from two experiments). Black squares = RIP-GP LCMV; white squares = RIP-GP LCMV + FTY720 day −2; red squares = RIP-GP LCMV + FTY d3. (I) C57BL/6 WT mice were treated daily with a sphingosine-1-phosphate antagonist [FTY720; 1 mg/kg body weight (bw)] starting from day −2 till day 3, from day 3 till day 8, or day 2 till day 8 of infection or left untreated. Mice were infected intravenously (i.v.) with 20 PFU of LCMV-WE. On day 9, tetramer (Tet) GP33-specific CD8+ T cells in iLNs were counted by flow cytometry (n = 6, pooled from two experiments). Black column = LCMV; gray column = LCMV + FTY720 (starting day −2 till day 3); red column = LCMV + FTY720 (starting day 3 till day 8); white column = LCMV + FTY720 (starting day −2 till day 8). Statistical significance was set at the level of P < 0.05 and was determined by Student’s t-test (A–D,G,I) or log-rank (Mantel–Cox) test (E,F,H). n.s., not significant; *P < 0.5; **P < 0.01; ***P < 0.001.