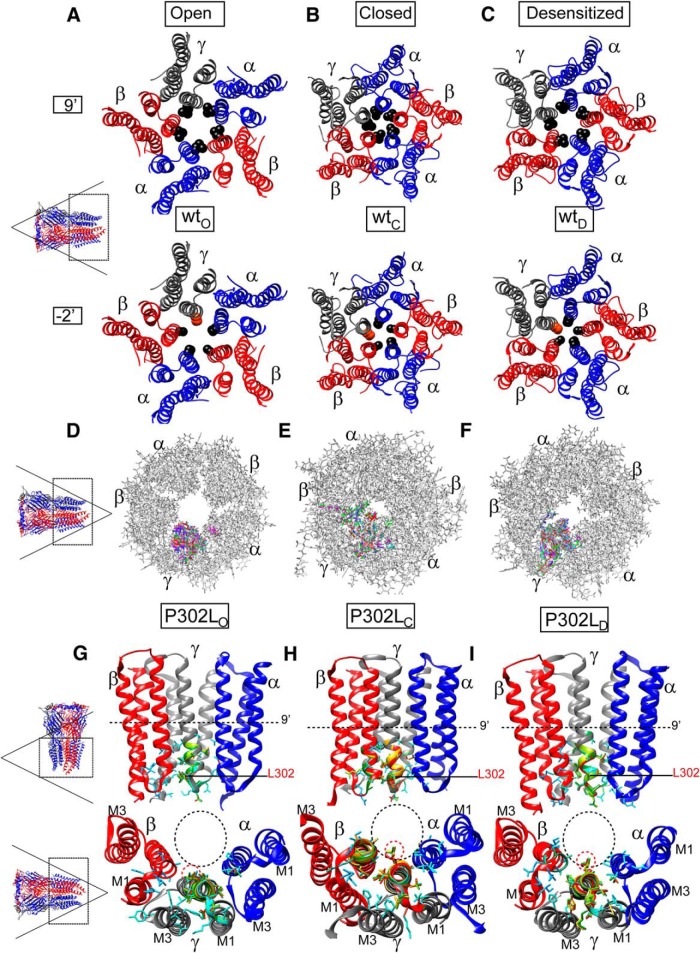
Figure 5.
The pore mutation P302L perturbed the conduction pathway of GABAA receptors. A-C, Transmembrane domains (M1 to M4) of 3D structural models of the α1β3γ2 GABAA receptor in the open (A), closed (B), and desensitized (C) conformational states were viewed from the extracellular side and displayed the β subunits in red, α subunits in blue, and γ subunit in gray. Side chains of the pore-lining residues at 9’(in black) and -2’ (P302 in orange, other residues in black) positions were shown within the ion channel pore of the receptor. D-F, Superimposed 10 best-scoring 3D transmembrane domains of GABAA receptors in the open (D), closed (E), and desensitized (F) states modelled between the initial wt and P302L mutated structures were viewed from the cytoplasmic side. The modelled structures were in stick representation. The wt GABAA receptor structure was in gray. The structural rearrangements in side chain residues that differ among the wt and the mutated structures (RMS ≥ 0.5 Å) were represented as a different color. G-I, Superimposed 10-best-scoring transmembrane domains of P302L structures in the open (G), closed (H), and desensitized (I) states were seen parallel to the membrane (top panels) and from the cytoplasmic side (bottom panels). Two subunits were removed for clarity. Perturbed neighborhood side chains within of 10 Å of L302 at the -2’ position were shown within the ion channel pore, and the structural perturbations that differ among the wt and mutated structures (RMS ≥ 0.5 Å) were represented in different colors. The wt-γ2 subunit structure was in gray. The channel gate at the 9’ position was represented as a dashed line in the top panels. The β and α subunits were in red and blue, respectively. In the bottom panels, dashed black circles represented the channel pore, and the location of the L302 residue was shown in red dashed circles. Lists of the residues perturbed by the L302 mutation were detailed in the text.