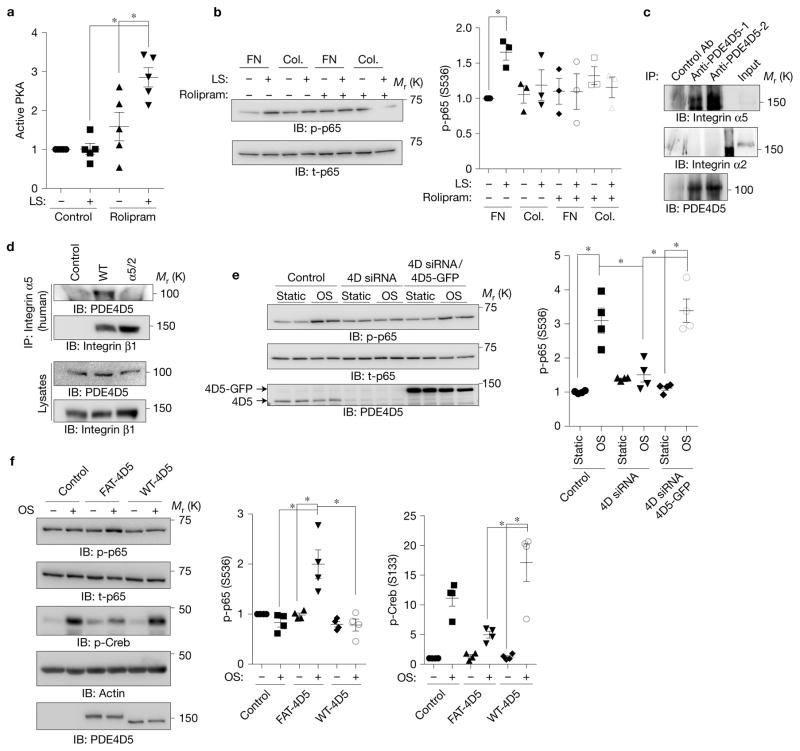
Figure 4.
Involvement of PDE4D in ECM-dependent inflammatory signalling. (a) BAECs on FN were treated with the PDE4 inhibitor rolipram (1 μM), sheared, and PKA activity assayed as in Fig. 1. (n = 5). (b) BAECs on FN or collagen (Col.) were treated with rolipram (1 μM) and assayed for NF-κB as before (n = 3). (c) HUVEC lysates were immunoprecipitated with two different PDE4D5 antibodies and western blots probed for integrin α5 or integrin α2. Similar results were obtained in three experiments. (d) BAECs expressing WT integrin α5 or the α5/2 chimaera were immunoprecipitated with human-specific integrin α5 antibody recognizing the extracellular region. Western blots were probed for PDE4D5 and for the integrin β1 subunit. Similar results were obtained in 4 experiments. (e) BAECs were transfected with siRNA against PDE4D and then rescued with control or PDE4D5-GFP retrovirus. Cells were subjected to oscillatory shear and NF-κB assayed as before (n = 4). (f) BAECs expressing WT PDE4D5 or FAT-PDE4D5 were plated on Matrigel for 5 h and subjected to oscillatory shear. NF-κB Ser536 phosphorylation and Creb Ser133 phosphorylation were measured by western blotting (n= 4). Data are represented as means ± s.e.m. *P < 0.05 by one-way ANOVA (a,e,f) or two-tailed t-test (b). In all panels n values represent independent experiments. Source data are provided in Supplementary Table 1. Unprocessed scans of blots are shown in Supplementary Fig. 6.