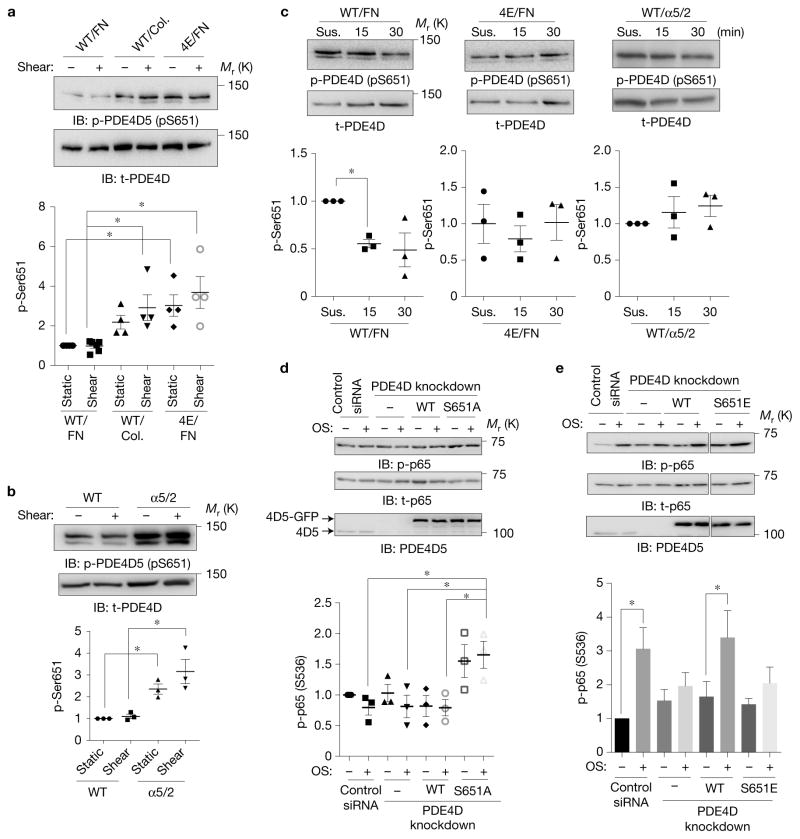
Figure 6.
ECM-dependent regulation of PDE4D phosphorylation. (a) BAECs expressing WT or the 4E mutant of PDE4D5 were plated on collagen or FN for 5 h and then sheared for 15 min. Cell lysates were probed for anti-pSer651-PDE4D (n = 4–6). Y axis values throughout the figure represent the fold change (relative to control). t-PDE4D, total PDE4D. (b) BAECs expressing WT integrin α5 or the α5/2 chimaera on FN were transfected with PDE4D5, and then sheared for 15 min. Ser651 phosphorylation was assayed by western blotting as in a (n= 3). (c) BAECs expressing PDE4D5 WT or the 4E mutant or chimaera cells expressing PDE4D5 WT were kept in suspension (Sus.) for 90 min and then replated on FN-coated dishes for the indicated times. Ser651 phosphorylation was assayed by western blotting (n = 3). (d,e) BAECs in which endogenous PDE4D5 was knocked down were reconstituted with WT, phospho-deficient S651A or phospho-mimetic S651E mutants. The cells were replated on collagen (d) (n = 3) or FN (e) (n = 6) and then subjected to oscillatory shear for 2 h. NF-κB activity was assayed as in Fig. 1. In all panels n values represent independent experiments. Data are represented as means ± s.e.m. *P < 0.05 by one-way ANOVA (a,b,d) or two-tailed t-test (c,e). Source data are provided in Supplementary Table 1. Unprocessed scans of blots are shown in Supplementary Fig. 6.