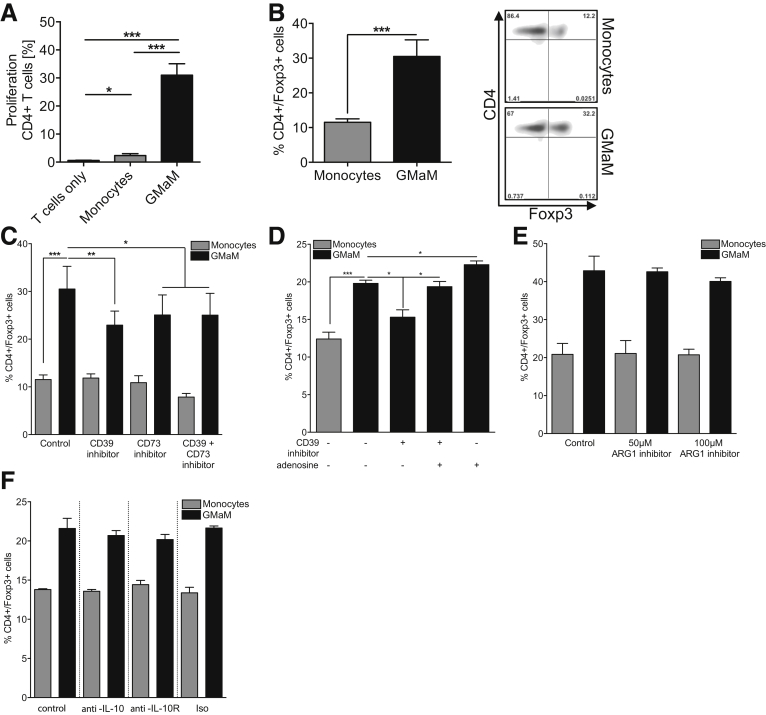
Figure 7.
Coculture of Granulocyte-macrophage colony-stimulating factor–activated monocytes (GMaM) with naive T cells led to proliferation and induction of Foxp3+CD4+T cells via adenosine/CD39 but not by arginase. (A) Naive carboxyfluorescein succinimidyl ester (CFSE)-labeled T cells were cocultured with respective monocytes (ratio 5:1, triplicates for each condition) or left alone as control. Cocultures were incubated for 5 days without further T-cell stimulation. Proliferation was evaluated as a decrease in CFSE fluorescence and quantified by analyzing the percentage of proliferating CD4+ T cells (n = 5). (B) T cells were cocultured with GMaM or control monocytes at a ratio of 5:1 (T cells/monocytes). Cells were stained for CD4 and Foxp3 expression and analyzed by flow cytometry. Dot plots (right panel) are representative for graph (left panel) that includes mean (± standard error of the mean) of five independent experiments. (C) To inhibit the function of CD39 and/or CD73, either CD39 inhibitor and/or CD73 inhibitor were added to the cocultures, respectively (n = 6). (D) To further evaluate the role of adenosine in regulatory T-cell induction, we added 10 μM adenosine to some cocultures (n = 4). (E) Arginase activity during cocultures was blocked using 50 μM or 100 μM ARG1 inhibitor (n = 4). (F) To inhibit the function of interleukin 10 (IL-10), either anti-IL-10 or anti-IL-10 receptor antibodies were used. (C–F) Cells were stained for CD4 and Foxp3 expression, and the percentages of double-positive T cells are shown. Statistical significance was determined by one-way analysis of variance with Bonferroni correction. *P < .05; **P < .01; ***P < .001.