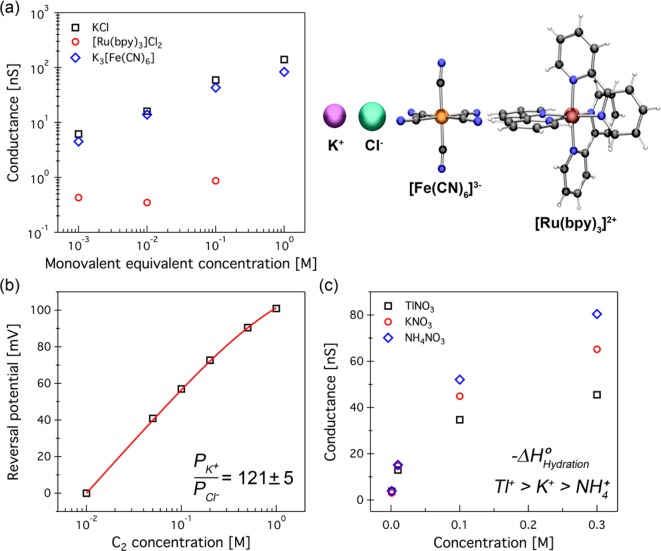
Figure 2.
Ion permeability through SWCNTs (Device 1). (a) Effect of ion size on conductance. Ionic conductance is plotted as a function of monovalent equivalent concentration of electrolytes with varying ion sizes. Comparing the conductance–concentration curves of KCl (black squares), K3[Fe(CN)6] (blue diamonds), and [Ru(bpy)3]Cl2 (red circles) suggests that cations are the major charge carriers through carbon nanotubes. (b) Reversal potential (Erev) as a function of KCl concentration in reservoir 2. Concentration of KCl in reservoir 1 was fixed at 10 mM, while in reservoir 2 [KCl] was varied between 10 mM and 1.0 M. Erev was always positive on the more dilute side confirming the cation selectivity of these channels. The solid line is the best fit of data according to the GHK equation yielding a permeability ratio of 121 ± 5 for cations over anions in KCl. (c) Effect of cation enthalpy of hydration. Conductance comparison among KNO3 (red circles), NH4NO3 (blue diamonds), and TlNO3 (black squares) electrolytes with various concentrations at a pH of ∼6.0. Cations with lower absolute value of enthalpy of hydration exhibit higher conductance.