Figure 1. Basic Pathophysiology of Hyperandrogenemia in the Polycystic Ovary Syndrome.
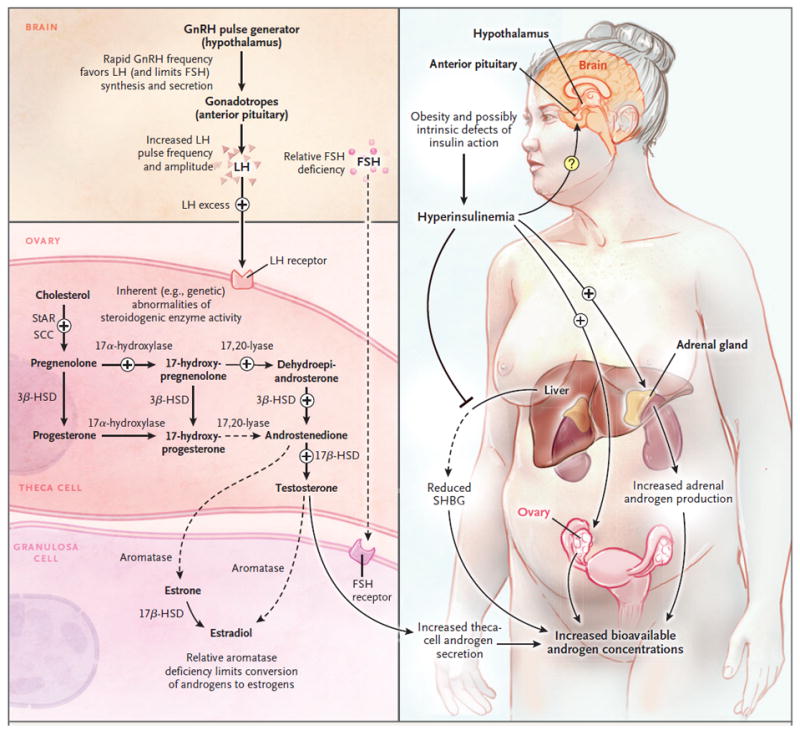
Since ovarian steroidogenesis requires gonadotropin stimulation, luteinizing hormone (LH) is a key factor in the hyperandrogenemia of the polycystic ovary syndrome.2 Progesterone is the primary regulator of gonadotropin-releasing hormone (GnRH) pulse frequency; however, in the polycystic ovary syndrome, the GnRH pulse generator is relatively resistant to the negative feedback effects of progesterone.3 This resistance to progesterone negative feedback appears to be mediated by androgen excess (since it is reversed by the androgen-receptor blocker flutamide).4 Resulting high GnRH pulse frequencies favor production of LH and limit production of follicle-stimulating hormone (FSH), which promote androgen production and interfere with normal follicular development. The polycystic ovary syndrome is associated with inherent abnormalities of ovarian (and adrenal) steroidogenesis: cultured ovarian theca cells from women with the polycystic ovary syndrome secrete excess androgens and precursors,5 and women with the polycystic ovary syndrome have exaggerated ovarian steroidogenic responses to gonadotropin stimulation.6 A recent study suggests that increased expression of a DENND1A splice variant drives a polycystic ovary syndrome–like steroidogenic phenotype in theca cells.7 The polycystic ovary syndrome is associated with insulin resistance — which is at least partly independent of obesity — and compensatory hyperinsulinemia.8 Hyperinsulinemia contributes to hyperandrogenemia in several ways: it augments LH-stimulated androgen production by ovarian theca cells, it potentiates corticotropin-mediated adrenal androgen production, and it inhibits hepatic synthesis of sex hormone–binding globulin (SHBG), which increases free testosterone levels. The precise effects of hyperinsulinemia on gonadotropin secretion remain unclear. 3ß-HSD denotes 3ß-hydroxysteroid dehydrogenase, 17ß-HSD 17ß-hydroxysteroid dehydrogenase, SCC cholesterol side-chain cleavage enzyme, and StAR steroidogenic acute regulatory protein.
