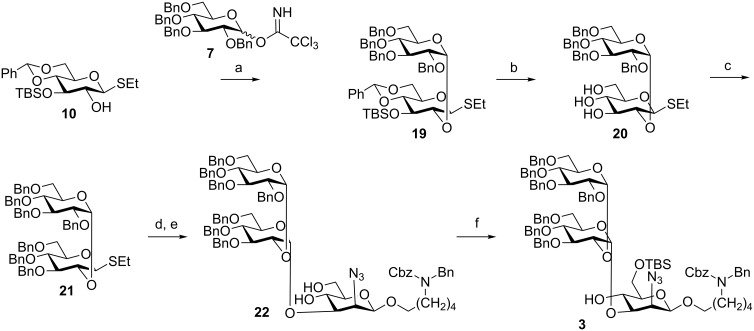
Scheme 4.
Synthesis of the reducing-end trisaccharide 3. Reagents and conditions: (a) TMSOTf, (CH3CH2)2O/CH2Cl2 (4:1), −20 °C, α/β = 4:1, 70%; (b) p-TsOH, CH3OH/CH2Cl2 (1:1), rt, 70%; (c) NaH, benzyl bromide, THF/DMF (1:1), 0 °C to rt, 90%; (d) 18, NIS, TfOH, (CH3CH2)2O/CH2Cl2, (4:1), −20 °C, 61%; (e) p-TsOH, CH3OH, rt, 90%; (f) TBSCl, imidazole, CH2Cl2, rt, 93%.