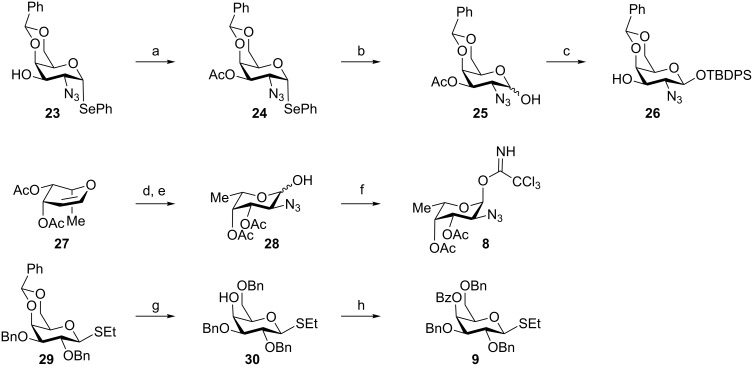
Scheme 5.
Synthesis of monosaccharide building blocks 8, 9 and 26. Reagents and conditions: (a) acetic anhydride, pyridine, CH2Cl2, rt, 18 h, 82%; (b) NIS, THF/H2O (1:1), rt; (c) 1) TBDPSCl, imidazole, DMF, rt; 2) NaOMe, MOH, rt, 68% over two steps; (d) (PhSe)2, BAIB, NaN3, CH2Cl2, rt, 24 h; (e) NIS, THF/H2O (1:1), rt, 80% over two steps; (f) CCl3CN, DBU, CH2Cl2, 0 °C to rt, 2 h, 72%; (g) TES, TFA, CH2Cl2, 0 °C, 6 h; (h) benzoyl chloride, pyridine, rt, 18 h, 80% over two steps.