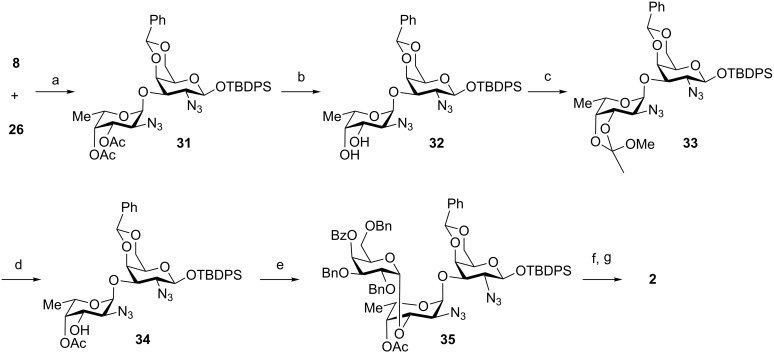
Scheme 6.
Synthesis of the non-reducing end trisaccharide 2. Reagents and conditions: (a) TMSOTf, CH2Cl2, −30 °C, 74%; (b) NaOMe (0.5 M in MeOH), MeOH, rt; (c) trimethyl orthoacetate, p-TsOH, toluene; (d) 80% AcOH, rt, 71% over three steps; (e) 9, NIS, TMSOTf, dioxane/toluene (3:1), −10 °C, 54%; (f) HF-pyridine, THF, 0 °C; (g) CCl3CN, DBU, CH2Cl2, 0 °C, 57% over two steps.