Abstract
This 12-month prospective randomized cluster trial of 20 dietitians in India compared usual care (UC) and evidence-based nutrition practice guideline (EBNPG) care for patients with type 2 diabetes mellitus. Baseline, 6-month, and 12-month data from 238 patients were analyzed. EBNPG implementation was evaluated using the Ottawa Model for Knowledge Transfer. EBNPG and UC groups achieved significant hemoglobin A1C improvements. EBNPG-treated participants were significantly more likely to meet low-density lipoprotein, high-density lipoprotein, and triglyceride goals at 6 or 12 months. Dietitian dropout, implementation barriers, and undetermined EBNPG intervention fidelity are limitations. Future research should assess barriers/supports and degree of EBNPG use.
Keywords: clinical nutrition, diabetes mellitus, dietetics outcomes, evidence-based guidelines, nutrition, nutrition care process
TODAY, quality health care requires consistently applied, evidence-based practice to achieve the most positive health care outcomes. Achieving high-quality, cost-effective nutrition care requires (1) developing standardized evidence-based nutrition practice guidelines (EBNPGs) and protocols and (2) evaluating patient-centered services based on thorough knowledge of patient problems, provider interventions, availability of time, and costs associated with achieving optimal patient outcomes.1–3
The Canadian Institutes of Health Research coined the term knowledge translation in 2000 to describe the specifics of how this occurs in health care and public health.4–6 The World Health Organization defines knowledge translation as “the synthesis, exchange, and application of knowledge by relevant stakeholders to accelerate the benefits of global and local innovation in strengthening health systems and improving people's health.”7 Research in knowledge translation has been used to create various models for how such translation might optimally occur.5 Specifically, the 6-step Ottawa Model for Knowledge Transfer includes a thorough assessment of the evidence-based innovation itself (development process and innovation attributes), potential adopters (their awareness, attitudes, knowledge/skill, concerns, and current practice), and the practice environment (patients, culture/social, structural, economic, and uncontrolled events).8 Based on the assessment, specific implementation intervention strategies and monitoring adoption procedures are developed and implemented, and outcomes of patients, practitioners, and the health care system are then collected and evaluated.4,8,9
Diabetes mellitus (DM) has become a major health problem worldwide. India, in particular, has a higher percentage of people with type 2 diabetes mellitus (T2DM) than any other country in the world.10 Estimates from the World Health Organization and the International Diabetes Federation indicate that the number of persons in India with diabetes could increase to 70 million by 2025 and to as high as 80 million by 2030.11–14 Those most at risk for T2DM are younger adults or even children, who tend to have greater waist circumference and thus greater central obesity, more visceral fat, and increased insulin resistance even at lower body mass indices (BMIs).10,12,15–20
Nutrition therapy has been and continues to be accepted as a cornerstone of diabetes management.21–25 Standards of medical care advocate individualized nutrition recommendations and instructions, such as medical nutrition therapy (MNT) provided preferably by a registered dietitian who is familiar with the components of diabetes-related nutrition management.26 Medical nutrition therapy services are defined in a statute as “nutritional diagnostic, therapy, and counseling services for the purpose of disease management which are furnished by a registered dietitian or nutrition professional ... pursuant to a referral by a physician.”27,28 The goals of MNT for patients with diabetes include achieving and maintaining (1) blood glucose close to or in the normal range, (2) a lipid and lipoprotein profile that reduces the risk for vascular disease, and (3) blood pressure close to or in the normal range.21,29,30
In India, dietitians who usually work as part of a health care team provide nutrition care for persons with DM. Diabetes mellitus nutrition practice guidelines do not exist in India, and the use of a standard protocol and provision of follow-up care at specific intervals are not common practices. Clients frequently pay for each medical/dietetic encounter, laboratory test, and other care as they receive the health care.
The Academy of Nutrition and Dietetics (the Academy) has been proactive in developing EBNPGs and the Nutrition Care Process (NCP) to continually improve the quality of nutrition care.31–36 The EBNPGs for persons with type 1 diabetes mellitus (T1DM) and T2DM were created by a credible US dietetics organization (the Academy); however, no local dietetic organization or group of physicians/diabetologists had evaluated, endorsed, or adapted these guidelines for use in India. The 2008 EBNPGs were recognized as being “evidence based” and were thus consistent with the prevailing health care focus on using evidence-based approaches to health care when this study was completed.37 However, dietitians in India were generally unfamiliar with the details of the process used to identify, evaluate, and synthesize the research into the guidelines. Although the use of the Academy EBNPGs has the potential to enhance patient health care outcomes, such use outside the United States during habitual office visits has not yet been widely studied.
The rising prevalence of DM in India and the potential for achieving improvements in patient outcomes as a result of implementing the Academy EBNPGs led to the Diabetes in India Nutrition Guidelines Study (DINGS), which was conducted from 2012 to 2013 throughout numerous Indian provinces. This small, randomized clinical trial (RCT) explored the impact of implementing the Academy EBNPGs in India on T2DM patient outcome and dietetics practice compared to usual care (UC).
MATERIALS AND METHODS
Aims and design
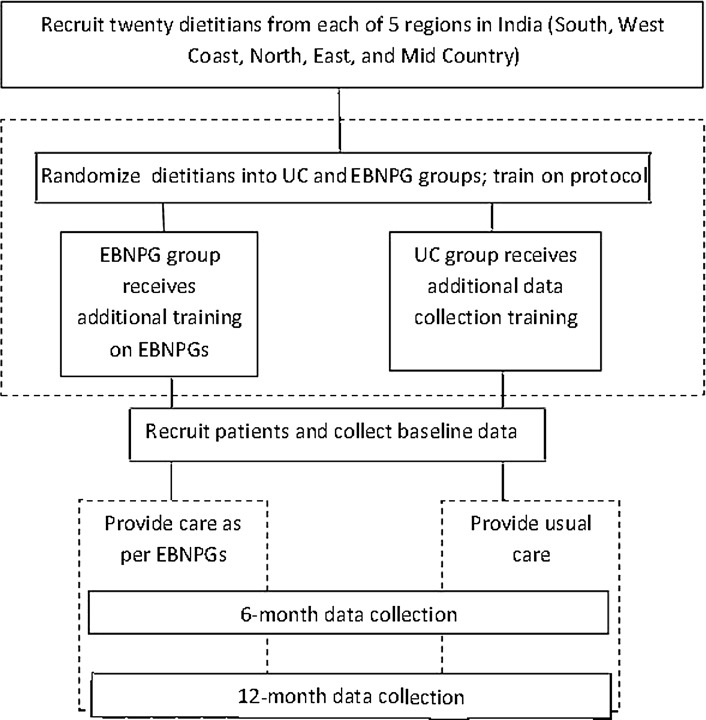
The 12-month prospective cluster RCT compared the outcomes of patients with T2DM who received dietitians' UC to patients who received EBNPG care (Figure 1).33 The Human Ethics Committee of Hebrew University of Jerusalem approved the protocol, as did the Alert Ethics Committee (EC-IEC) of Snehal Hospital in Thane, Maharashtra, India, and 14 local ethics committees.
Figure 1.
DINGS Research Design. DINGS indicates Diabetes in India Nutrition Guidelines Study; EBNPG, evidence-based nutrition practice guideline; UC, usual care.
Recruitment
Dietitian recruitment
Dietitians with at least 2 years of postprofessional degree experience working at diabetes centers or hospitals specializing in diabetes were recruited from the North, South, Central, East, and West regions of India. To be eligible, dietitians had to meet the following criteria: hold a national dietetics credential (eg, Indian or others, such as a US or Canadian registered dietitian credential), have a large enough patient population to recruit 4 to 6 “new” patients with T2DM per week into the study, be associated with a diabetologist who could provide medical oversight for the study, provide a letter of support from an immediate supervisor and diabetologist indicating that MNT visits could be provided without cost to patients during the research study, have computer access with Microsoft Internet Explorer 6.0 or higher and a browser enabled for Java, have an Internet connection, be able to obtain ethics approval for the study, have the capability to adjust counseling schedules for patients to change appointment length and frequency to match guideline recommendations and support data collection, be able to travel to a central location for face-to-face training, and commit to attending webinars and telephone calls during the study.
The dietitians completed an extensive application and were interviewed by an in-country research coordinator. Twenty-four dietitians who met the eligibility requirements were randomized into either UC or EBNPG care groups and attended the initial training. Twenty dietitians were able to receive institutional review board approvals to participate and 12 of them contributed data.
Patient recruitment
Dietitians were asked to recruit up to 30 patients from their current referrals. Patient recruitment and consent materials were translated into 11 different local Indian dialects/languages. Patients were eligible for the study if they (1) were older than 19 years with a medical diagnosis of T2DM, (2) were not receiving injectable insulin therapy, (3) had not been seen by a dietitian for 12 months prior to recruitment, and (4) agreed to return for follow-up laboratory tests at 6 and 12 months. Patients were not eligible to participate if they currently had a medical diagnosis of end-stage renal disease, cardiovascular accident, coronary artery disease, myocardial infarction, congestive heart failure, chronic obstructive pulmonary disease, or depression; if they had a current diagnosis and treatment of cancer or cognitive limitations; or if they had an unplanned surgery with an overnight hospital stay within a week of recruitment.
Data collection
Each dietitian coordinated with local laboratory staff to collect and ship patient blood samples to the same centralized laboratory for processing. Data were reported directly from the centralized laboratory to both researchers and to study dietitians for age, gender, hemoglobin A1C (HbA1C), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides.
Other data collected during the NCP were entered by dietitians into an online data portal (the DINGS portal) for height, weight, and details of the nutrition intervention. A generic template with basic information was developed for UC data entry. A template that followed the T2DM EBNPGs was developed for the EBNPG group.
Patient sample
Data from the centralized laboratory were available for 239 of the recruited patients, and additional patient data were available from the DINGS portal for 176 of the 239 participants. Follow-up data were available at either 6 months or 12 months for 133 patients. Data were available for baseline and both 6 months and 12 months for 97 patients, for only baseline and 6 months for 21 patients, and for only baseline and 12 months for 15 patients. Age, gender, and laboratory parameters (HbA1C, total cholesterol, HDL, LDL, and triglycerides) were not significantly different for the patients who returned for the 6-month data collection compared with those who were lost to follow-up.
Intervention
Training on the research protocol and data collection procedures was provided to all dietitians. The UC dietitians continued their habitual practice; however, they asked patients to return at 6 months and 12 months for follow-up laboratory testing. The EBNPG dietitians were given additional training on the (1) NCP, (2) standardized language, (3) T1DM and T2DM EBNPGs (summarized in Table 1), (4) use of the Nutrition Progress Report Forms included in the companion toolkit for the T2DM EBNPGs, (5) use of self-monitoring blood glucose (SMBG) equipment, and (6) motivational interviewing. Table 1 also identifies which of the EBNPG recommendations required a change from UC provided by dietitians in India. EBNPG dietitians were given glucometers and strips to provide to their patients to facilitate SMBG consistent with the EBNPGs and were asked to modify their nutrition care to be consistent with the EBNPGs.
Table 1. Summary of Recommendations in EBNPGs for Persons With Type 1 and 2 Diabetes Mellitusa.
| Step of Nutrition Care Process | Recommendationb | Strength of Recommendationc and Categoryd | Guideline | Comparison to Usual Care in Indiae |
|---|---|---|---|---|
| Number and length of initial series of MNT encounters | Strong, imperative | 3-4 encounters (45-90 min) within 3-6 mo after referral | Different | |
| MNT long-term follow-up encounters | Strong, imperative | Dietitian determined, regular sessions sustained positive outcomes | Different | |
| Nutrition assessment | Nutrition assessment | Strong, imperative | Assess food intake, medication, metabolic control, anthropometric measurement, and physical activity | Usually same |
| Assessment of glycemic control | Strong, imperative | Assess glycemic control and focus on achieving target blood glucose levels | Usually same | |
| Assess relative importance of weight management | Strong, conditional (for those who are overweight or obese) | Modest weight loss may improve insulin resistance; however, long-term impact inconsistent | Usually same | |
| Nutrition intervention | Intervention options | Strong, imperative | Implement MNT selecting from a variety of interventions, education, and counseling sensitive to personal needs and based on willingness to change and ability to make changes | Varied, usually relied on nutrition education vs nutrition counseling |
| Macronutrient percentages | Strong, imperative | Macronutrients based on national dietary guidelines | Usually same | |
| Carbohydrate intake consistency | Strong, conditional (persons with medication) | Keep meal and snack carbohydrate intake consistent on a day-to-day basis | Usually same | |
| Sucrose intake | Strong, conditional (persons who choose to eat foods with sucrose) | Not more than 10%-35% of total energy substituted for other carbohydrate containing foods | Usually same | |
| Nonnutritive sweeteners | Fair, conditional (persons who choose to consume nonnutritive sweeteners) | Advise to stay less than average daily intakes; no impact on glycemic control | Different; not usually addressed | |
| GI | Fair, conditional (when GI is proposed as a method of meal planning) | Advise that GI research reports mixed results on HbA1C | Different | |
| Fiber intake and glycemia | Strong, imperative | 44- to 50-g fiber is reported to improve glycemia; however, unsure if this level is feasible | Same | |
| Fiber intake and cholesterol | Strong, imperative | 25- to 30-g fiber (emphasizing soluble) can reduce cholesterol | Same | |
| Protein intake and normal renal function | Fair, conditional (persons with normal renal function) | Maintain usual intake of 20%-25% of energy from protein | Same | |
| Blood glucose monitoring | Fair, conditional (persons on nutrition therapy alone or in combination with glucose-lowering medication) | Frequency and timing of blood glucose monitoring dependent on DM goals and therapies and incorporated into diabetes education programs | Different; not usually addressed in T2DM | |
| CVD and cardioprotective nutrition interventions | Strong, imperative | Reduction in saturated and trans fat and dietary cholesterol and interventions to reduce blood pressure | Different | |
| Diabetes and weight management | Fair, conditional | Unclear that weight loss along will improve glycemic control | Same | |
| T2DM and physical activity | Strong, conditional | 50-90 min of accumulated moderate-intensity aerobic physical activity per week as well as resistance/strength training 3 times per week | Different | |
| Coordination of care | Imperative, consensus | Coordinate care with interdisciplinary team approach | Different | |
| Monitoring and evaluation | Monitoring and evaluation | Strong, imperative | Monitor and evaluate food intake, medication, metabolic control, anthropometric measures, and physical activity | Different; many times not able to have follow-up visits to monitor |
| Evaluation of glycemic control | Consensus, imperative | Use blood glucose monitoring results to evaluate effectiveness of MNT | Different; many times not able to have follow-up visits to monitor |
Abbreviations: CVD, cardiovascular disease; DINGS, Diabetes in India Nutrition Guidelines Study; EBNPG, evidence-based nutrition practice guideline; GI, glycemic index; HbA1C, hemoglobin A1C; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MNT, medical nutrition therapy; SMBG, self-monitoring blood glucose; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; UC, usual care.
aAdapted with permission from American Dietetic Association31 and Academy of Nutrition and Dietetics.40
bSelected recommendations that apply to the DINGS population. Other recommendations not applicable to this population are carbohydrate intake and insulin dose adjustment, protein intake and nephropathy, protein intake and late-stage nephropathy, frequency of blood glucose monitoring, possible need for continuous glucose monitoring or more frequent SMBG, T1DM and physical activity, physical activity and insulin/insulin secretagogue use).
cRecommendations are rated as Strong, Fair, Weak, Consensus, or Insufficient Evidence. Strong: Good/strong evidence identified that benefits of following the recommendation exceed the harms; practitioners should generally follow the recommendation unless a clear and compelling rationale for alternative approach is present. Fair: Good/fair evidence identified that benefits of following the recommendation exceed the harms; practitioners should generally follow the recommendation but remain alert to new information and sensitive to patient preferences. Consensus: No studies are available, conclusion based on expert opinion; practitioners should be flexible in deciding whether to follow, and patient preferences should be a substantial influencing role.
dRecommendations fall into 2 categories: Imperative or conditional. Imperative recommendations are broadly applicable to the target population, whereas conditional recommendations apply only to a specific situation or population.
eComparison to Usual Care in India: Same indicates that all dietitians agreed that this was the same as their usual practice, usually same indicates that few dietitians did not indicate it was the same of their usual practice, and different indicates that all dietitians agreed that this was different from their usual care. Dietitians attempted to implement all applicable guidelines.
Statistical analysis
Data were analyzed using SAS software (version 9.2; SAS Institute, Cary, North Carolina). Differences between baseline, 6-month, and 12-month data were computed for each patient. Statistical significance of differences between the baseline demographics for EBNPG and UC groups and amount of change at 6 months and 12 months were computed and compared using 2-sample t tests for individual levels. Chi-square tests were used to test for significance for the percentage of patients achieving goal at the end of the 12-month period. Significance of the differences between within-group baseline and 6-month values and between baseline and 12-month values was established using the paired-samples t test. Because of the skewed distribution of triglyceride values, these were log-transformed before analysis. Descriptive statistics were computed for other variables when available in the EBNPG group only, for example, percentage of patients with a given nutrition diagnoses or receiving a specific intervention.
Findings
The number of subjects, mean differences, and standard deviations for laboratory parameters for the EBNPG and UC groups are shown in Table 2. Despite the age being statistically different at baseline, the outcomes are unlikely to be affected by the 4-year age difference between UC and EBNPG.
Table 2. Comparison of Baseline Demographics for the EBNPG Group and the UC Group.
| Parameter | Total | EBNPG Group | UC Group | P Value (EBNPG vs UC)a | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | Min | Max | n | Mean ± SD | n | Mean ± SD | ||
| All | 239 | 85 | 154 | ||||||
| Men | 143 (60%) | 46 (54%) | 97 (63%) | ||||||
| Women | 96 (40%) | 39 (46%) | 57 (37%) | ||||||
| Age, y | 219 | 46.3 ± 9.5 | 25 | 69 | 68 | 43.6 ± 9.3 | 151 | 47.6 ± 9.4 | .004 |
| Height, cm | 170 | 162.4 ± 9.4 | 140 | 180 | 51 | 161.0 ± 9.8 | 119 | 163.0 ± 9.3 | .20 |
| Weight, kg | 170 | 70.4 ± 12.9 | 45 | 116 | 51 | 69.3 ± 10.2 | 119 | 70.9 ± 14.0 | .43 |
| Body mass index | 170 | 26.6 ± 4.0 | 20 | 43 | 51 | 26.8 ± 3.2 | 119 | 26.6 ± 4.3 | .79 |
| HbA1C | 237 | 8.8 ± 2.4 | 4.6 | 16.0 | 84 | 8.7 ± 2.3 | 153 | 8.9 ± 2.5 | .63 |
| Total cholesterol | 238 | 186.2 ± 40.8 | 75 | 308 | 84 | 185.5 ± 42.9 | 154 | 186.6 ± 39.7 | .85 |
| LDL cholesterol | 238 | 113.4 ± 31.6 | 18 | 237 | 84 | 113.6 ± 31.7 | 154 | 113.3 ± 31.6 | .95 |
| HDL cholesterol | 238 | 39.1 ± 10.0 | 19 | 88 | 84 | 37.9 ± 8.8 | 154 | 39.8 ± 10.5 | .14 |
| Triglycerides | 238 | 191.5 ± 210.3 | 44 | 2714 | 84 | 205.8 ± 290.1 | 154 | 183.7 ± 150.7 | .32 |
Abbreviations: EBNPG, evidence-based nutrition practice guideline; HbA1C, hemoglobin A1C; HDL, high-density lipoprotein; LDL, low-density lipoprotein; UC, usual care.
aP values were calculated using 2-sample t test.
Table 3 shows the differences in parameters between baseline and 6 months and baseline and 12 months by group. Except for total and LDL cholesterol at 6 months, all parameters in the EBNPG group were significantly improved from baseline at both 6 months and 12 months; however, in the UC group, changes from baseline were only statistically significantly different for HbA1C. Only triglycerides improved significantly more in the EBNPG group compared with the UC group at both 6 and 12 months.
Table 3. Comparing Average Change From Baseline at 6 Months and 12 Months for the ENBPG Group vs the UC Group.
| Parameter and Measurement Period | EBNPG Group | UC Group | P Significance of Difference Between EBNPG and UC | ||
|---|---|---|---|---|---|
| n | Mean ± SD Change From Baseline | n | Mean ± SD Change From Baseline | ||
| HbA1C, mg/dL | |||||
| 6 mo | 37 | −1.69 ± 1.89b | 81 | −1.30 ± 2.44b | .39 |
| 12 mo | 35 | −1.03 ± 1.94c | 77 | −1.02 ± 2.49b | .99 |
| Body mass index | |||||
| 6 mo | 23 | −0.78 ± 1.45d | 45 | −0.27 ± 1.58 | .20 |
| 12 mo | 17 | −0.95 ± 1.87d | 42 | −0.52 ± 1.95 | .43 |
| Total cholesterol, mg/dL | |||||
| 6 mo | 37 | −11.0 ± 40.9 | 81 | −6.0 ± 38.6 | .51 |
| 12 mo | 35 | −14.9 ± 30.0c | 77 | −6.7 ± 36.3 | .25 |
| LDL cholesterol, mg/dL | |||||
| 6 mo | 37 | −0.8 ± 30 | 81 | −4.5 ± 31 | .54 |
| 12 mo | 35 | −11 ± 20c | 77 | −5.6 ± 30 | .25 |
| HDL cholesterol, mg/dL | |||||
| 6 mo | 37 | +2.0 ± 4.3c | 81 | +0.7 ± 6.5 | .18 |
| 12 mo | 35 | +1.6 ± 4.9c | 77 | +0.2 ± 9.6 | .30 |
| Triglyceride, mg/dLa | |||||
| 6 mo | 37 | −87 ± 348c | 81 | −3 ± 89 | .01 |
| 12 mo | 35 | −74 ± 224c | 77 | −4 ± 85 | .01 |
Abbreviations: EBNPG, evidence-based nutrition practice guideline; HbA1C, hemoglobin A1C; HDL, high-density lipoprotein; LDL, low-density lipoprotein; UC, usual care.
aSignificance established after log transformation with 2-sample t test.
bP ≤ .001 significance of change from baseline within group using paired-samples t test.
cP ≤ .01 significance of change from baseline within group using paired-samples t test.
dP ≤ .05 significance of change from baseline within group using paired-samples t test.
Table 4 summarizes the percentage of patients who met goal at 12 months. Patients in the EBNPG group were significantly more likely to meet goals for LDL, HDL, and triglycerides than patients in the UC group.
Table 4. Percentage of Patients at Expected Outcome or Ideal-Goal Value34 at 12 Months.
| Parameter | Group | n | Met Expected Outcome or Ideal or Goal Value, %a | Not Meeting Expected Outcome or Ideal or Goal Value, % | Significance of Difference Between Groupsb |
|---|---|---|---|---|---|
| HbA1C | EBNPG | 35 | 40.0 | 60.0 | .52 |
| UC | 77 | 33.8 | 66.2 | ||
| Total cholesterol | EBNPG | 35 | 74.3 | 25.7 | .11 |
| UC | 77 | 58.4 | 41.6 | ||
| LDL cholesterol | EBNPG | 35 | 65.7 | 34.3 | .003c |
| UC | 77 | 36.4 | 63.6 | ||
| HDL cholesterol | EBNPG | 35 | 74.3 | 25.7 | .047d |
| UC | 77 | 54.6 | 45.5 | ||
| Triglycerides | EBNPG | 35 | 82.9 | 17.1 | .003c |
| UC | 77 | 53.3 | 46.8 |
Abbreviations: EBNPG, evidence-based nutrition practice guideline; HbA1C, hemoglobin A1C; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NS, not significant; UC, usual care.
aExpected Outcomes or Ideal-Goal values-goals were defined by 2011 Diabetes Mellitus Toolkit (28) used in the research: HbA1C, <7 mg/dl; LDL cholesterol, <100 mg/dL; total cholesterol, any decrease from baseline; HDL, no change or increase; triglyceride, decreased or no change. (Note. 2015 Guidelines have been published, but the 2011 Toolkit is the most current toolkit available at www.eatright.org.)
bSignificance calculated using chi-square tests.
cSignificance of P ≤ .01.
dSignificance of P ≤ .05.
EBNPG dietitians also recorded details of their nutrition care using the Nutrition Care Process and Terminology (NCPT) in the 4 steps of the NCP. Data were available for 35 patients for 1 to 7 visits. Of the 4 dietitians who submitted data at 12 months, only 2 had some patients who received nutrition care appointments at the frequency and duration recommended in the EBNPGs.
EBNPG group nutrition care
Nutrition diagnoses
Of the 3 broad categories/domains (Behavioral/Environmental, Intake, and Clinical38), 48% of the nutrition diagnoses recorded were from the Behavioral/Environmental domain and recorded more than 5 times:
physical inactivity,
limited adherence to nutrition-related recommendations, or
inability or lack of desire to manage self-care.
Forty-four percent of the nutrition diagnoses recorded were from the Intake domain with the following being recorded more than 5 times:
inappropriate intake of types of carbohydrate (later changed to less than optimal intake of types of carbohydrate),
inconsistent carbohydrate intake,
inadequate fiber intake, and
excessive carbohydrate intake.
Nine percent of the nutrition diagnoses reported were from the clinical domain and only overweight/obesity was used more than 5 times. Other nutrition diagnoses used 2 to 5 times were inability or lack of desire to manage self-care, inadequate fat intake, undesirable food choices, involuntary weight gain, food and nutrition-related knowledge deficit, excessive energy intake, excessive fat intake, and disordered eating pattern.
The nutrition diagnoses evaluated as being resolved more than 5 times were excessive carbohydrate intake, inadequate fiber intake, and inconsistent carbohydrate intake. Other nutrition diagnoses resolved between 2 and 5 times were physical inactivity, food and nutrition-related knowledge deficit, excessive energy intake, inability or lack of desire to manage self-care, limited adherence to nutrition-related recommendations, overweight/obesity, excessive fat intake, undesirable food choices, self-monitoring deficit, involuntary weight gain, inappropriate intake of types of carbohydrate, and inadequate fluid intake.
Nutrition intervention
The most common forms of nutrition education reported were recommended modifications, priority modifications, nutrition relationship to disease, and results interpretation. All patients received nutrition education handouts, such as meal plans, exchange lists, diabetes instructions, or daily routines. Other nutrition intervention strategies reported included (in descending order) motivational interviewing, goal setting, and self-monitoring.
Nutrition monitoring and evaluation
The most commonly used indicators for monitoring and evaluating patient progress were HbA1C, BMI, weight, HDL cholesterol, weight change, LDL/HDL ratio, and LDL cholesterol.
Changes in HbA1C
Improvements in HbA1C were similar to or better than other studies evaluating changes in HbA1C in patients who received nutrition care from dietitians. At 6 months, Franz et al,22 Lemon et al,24 and Trostler et al39 reported decreases in HbA1C of 0.9 mg/dL, 1.7 mg/dL, and 1.4 to 1.5 mg/dL, respectively. Decreases of 1.69 mg/dL and 1.30 mg/dL were noted at 6 months in the DINGS EBNPG and UC groups, respectively. At 12 months, Trostler et al39 reported a decrease of 1.5 to 1.6 mg/dL, compared with reductions of 1.03 and 1.02 mg/dL in the DINGS EBNPG and UC groups, respectively.39
Dietitian attrition
There was considerably more attrition at the dietitian level in the EBNPG group, where there was greater demand for influencing the health care system. Although 9 of the 12 dietitians randomized to the UC group were successful in providing at least 1 patient with data from baseline and 12 months, only 4 of the 12 randomized to the EBNPG group were able to recruit and retain patients. The UC group did not routinely have any standard that specified the desirable number of follow-up visits. Providing care according to the EBNPGs would have resulted in up to 5 nutrition consultations in the first year and at least 1 nutrition consultation each year thereafter. However, only 2 of the 4 dietitians and 9 of the 35 patients in the EBNPG group met this level of participation.
DISCUSSION
While the EBNPG group achieved significant improvements from baseline at 12 months in all parameters, for example, HbA1C, BMI, total cholesterol, LDL cholesterol, HDL cholesterol, and triglyceride, the UC group also achieved a significant improvement in HbA1C. However, since the UC group also reported improvements in all parameters, only 1 parameter, triglyceride, was significantly more improved in the EBNPG when compared to the change in the UC group.
One of the assumptions made during this study was that with screening and training of the participating dietitians, they would be able to effectively implement the Academy EBNPGs into their practice. However, this study identifies that the challenges encountered in changing practice for patients enrolled in this study within an existing health care system may require more significant resources and a different level of support than what was incorporated into this project. Although application screenings and interviews were accomplished and the in-country research collaborator assumed the role of coordinating requests for assistance and communication to support data collection, extensive on-site support throughout the project focusing on assisting the dietitians in identifying and addressing barriers to change at the health care system level was not provided. Data and information were collected from dietitians throughout the research in a variety of ways, for example, through the initial questionnaires, training evaluation forms, e-mails, and verbal feedback during the on-line webinars. The following discussion is based on this information and views the implementation process by using a knowledge transfer model.
Implementation guided by Ottawa Model of Research Use
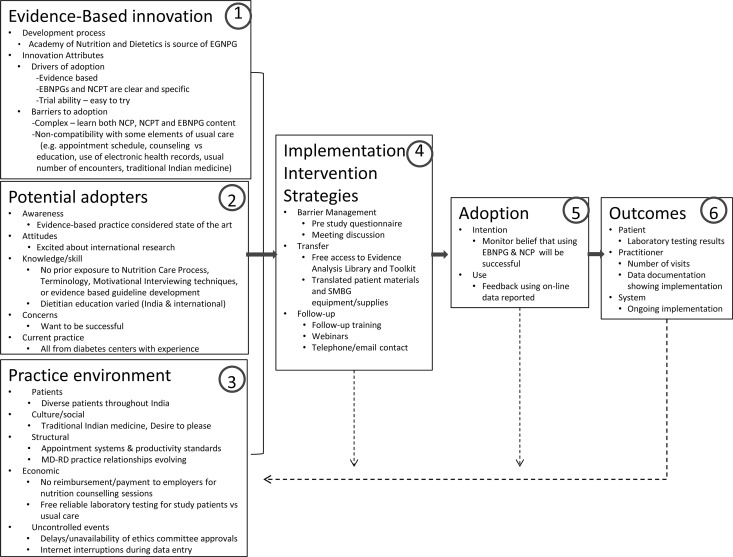
The Ottawa Model of Research Use5 identifies components thought to be important to successful adoption of innovations. Figure 2 shows how this model applies to the DINGS project, where the innovation being implemented is the EBNPGs for T1DM and T2DM. The following discussion of each box in the model in Figure 2 includes both components that promoted adoption and barriers to adoption.
Figure 2.
DINGS model of adopting innovations. DINGS indicates Diabetes in India Nutrition Guidelines Study; EBNPG, evidence-based nutrition practice guideline; NCP, Nutrition Care Process; NCPT, Nutrition Care Process and Terminology; SMBG, self-monitoring blood glucose. Adapted from the “Ottawa Model of Research Use: A Framework for Adopting Innovations” with permission from the National Collaborating Centre for Methods and Tools5 (available at http://www.nccmt.ca/registry/view/eng/65.html. Copyright © 2006 The Alliance for Continuing Medical Education, the Society for Medical Education, the Society for Academic Continuing Medical Education, and the Council on CME, Association for Hospital Medical Education).
Evidence-based innovation (Figure 2, Box 1)
Factors affecting the successful implementation of an innovation include the perceived credibility and validity of the development process as well as the characteristics of the innovation itself.
Graham et al8 identified key characteristics of innovations, stating that an innovation should be perceived to be useful (advantage over the current system), easy to use or do, easy to try, clear and specific about what is expected, evidence based, and compatible with current practice, norms, and values and not require a change in existing practice; innovation should also allow for reinvention (see Figure 2, Box 1)
Innovations that are clear and explicit tend to have higher adoption. The EBNPGs and NCPT are explicit. Each of the more than 300 terms was supported by an evidence-based definition and description of the appropriate use of the term. The EBNPGs have a detailed toolkit that describes the elements of care to be provided, and a sizeable (≥400 pages) reference manual describing the approach and terms to be used in the NCP was developed (available by subscription at https://ncpt.webauthor.com/).
Attributes that made implementing the EBNPGs more challenging included the complex nature of the terminology used to document the nutrition care and the fact that the EBNPG dietitians were requested to implement the EBNPGs rather than just try them out (limited trial ability). It became evident that some elements of the EBNPGs were not compatible with existing health care systems, values, norms, and practices in India. The number and length of appointments recommended by the EBNPGs were different from the care being provided to other patients and affected productivity. The practice of focusing on nutrition counseling and education was not familiar to all dietitians. The use of electronic systems to record details of nutrition encounters was also not familiar to the majority of dietitians.
Potential adopters (Figure 2, Box 2)
Awareness, attitudes, knowledge, and current practice of the EBNPG dietitians believed to affect adoption are reflected in Figure 2, Box 2. The supervisors and dietitians invited to participate in the DINGS project initially reflected a high level of excitement about being involved in an international project; however, they had limited awareness of the extent of the time and energy commitment of such a project. All of the dietitians were experienced in treating patients with diabetes, were employed in diabetes centers, were viewed as experts in their field, and expressed commitment to be successful in the research project. Few dietitians had experience with teaching or using blood glucose monitoring equipment, none had prior exposure to the NCP concept and standardized language, and participating dietitians had varying knowledge of specific nutrition counseling theories and strategies (eg, motivational interviewing). The initial dietetics education/training varied, depending on their dietetics education programs in England, India, and Canada.
Practice environment (Figure 2, Box 3)
Patients, culture/social, structure, economy, and other uncontrolled events influencing implementation are shown in Figure 2, Box 3. Patients were diverse and were recruited throughout India. Physician and dietitian relationships are evolving and the dietitians' ability to implement the EBNPGs was influenced by both their physician's awareness and acceptance of the content of the EBNPGs as well as by traditional medicine practice. Current emphasis on using SMBG levels may lead to different advice from physicians and dietitians in the interpretation of how to best use the SMBG results. Although dietitians and supervisors reviewed the key factors of the practice environment that would be critical to their participation, they were overly optimistic about their ability to influence the appointment schedule, adjust productivity standards to allow adequate time for patient care as well as data entry, and obtain ethics committee reviews. The research study provided free laboratory testing and provided the results directly to the dietitian to use in patient care. Funds were not provided to dietitian employers to compensate for dietitian research time. Ethics review took up to 18 months to obtain at the local institutional level. Reliable uninterrupted Internet service was not available at work or at home; thus, data entry through the Web site was compromised. Although the dietitians attempted to influence their environment, they were reluctant to ask for additional assistance from the researchers.
Implementation intervention strategies (Figure 2, Box 4)
Multiple strategies for barrier management, knowledge transfer, and follow-up are summarized in Figure 2, Box 4. Barrier management included using the preselection application to identify critical factors (eg, Internet availability, computer capabilities, flexibility in appointment schedules, and amount of time for nutrition care and data entry). These were addressed in recruitment and in the initial training; however, no routine system to monitor these factors was established after the initial training and during the implementation phase. The primary strategy used was providing hands-on education/training (initial and follow-up) and access to resources (eg, access to the Academy Evidence Analysis Library, patient handouts in 11 Indian dialects, and SMBG equipment and supplies). Participation in follow-up training/webinars and telephone contact was marginal. E-mail progress reports were provided; however, e-mails, telephone contacts, and webinars were not effective for discussing the patient recruitment and implementation barriers being encountered.
Adoption (Figure 2, Box 5)
Both intention and actual use are considered during the implementation phase, as shown in Figure 2, Box 5. The researchers monitored the frequency of data entry and recruitment and provided feedback about the number of patients recruited and the number of visits. However, evaluating the actual care being provided during this time was not possible due to lack of timely upload of data being collected. Although the telephone contacts, online discussion on the DINGS portal, and e-mail communication continued to reflect the dietitians' willingness to participate, the actual participation and data entry were not reflective of their stated intentions. The long delay in some ethics committee reviews tempered the dietitians' initial enthusiasm and, in some cases, the work environment/situation changed during this time period, precluding their continued participation.
Outcomes (Figure 2, Box 6)
The outcomes can be reflected at 3 different levels: patient (which is what is reflected by the hypothesis of this study), dietitian, and system (shown in Figure 2, Box 6). Patient-level data for laboratory test results were not dependent on dietitian data entry into the online system because they were directly incorporated into a report sent directly to researchers and, hence, are most complete. However, the data for other patient outcomes (weight, knowledge, behavior changes) and the specific details about nutrition care necessary to measure the fidelity of EBNPG implementation were dependent on dietitian data entry and were much less complete. Dietitians did not systematically complete data entry as each patient visit occurred, so data about ongoing implementation of EBNPGs during the study by the intervention group were unavailable.
Implications for future EBNPG guideline implementation in India
The implementation of the EBNPGs was perceived to be part of a research project. Applying the model at the national level would be useful. Verifying or adding to the factors promoting the use of EBNPGs and the barriers to implementation from the national perspective may lead to identifying more robust implementation strategies and data collection efforts. Perhaps one of the first steps would be an initiative to create an enhanced overall awareness of the benefits and use of evidence-based practice. This can occur at the national level through professional societies, government regulations, and professional meetings of dietitians and other health care professionals, with emphasis on recognizing what evidence-based practice guidelines are, illustrating their benefits, and identifying how to make using EBNPGs part of daily practice. In addition, it would need to be incorporated into the education of dietitians.
Implications for future research
The limitations of this study include difficulties in gaining ethics committee approvals at local hospital level, lack of additional compensation for dietitians for research participation (beyond travel expenses and training certificates), instability of electricity and Internet connections, lack of a robust mechanism and funding for in-country investigator to travel to dietitian sites during the research (not just at the beginning to recruit dietitians), and drop-out of dietitians and subsequently patients, particularly in EBNPG group where the burden of research was greater and magnitude of change in practice was greater.
Elaboration of the significant limitations of this study can provide insights into the feasibility of future international practice-based research efforts. The feasibility of practice-based research may need to be rethought in situations where the prevailing health care culture is not oriented toward documentation of outcomes using electronic methods. Although the concept of collecting data as part of routine patient care may be the optimal way to truly investigate how effective an intervention is in actual practice, this may only be feasible if all of the data needed for the research are already being collected in existing data systems and recorded across all sites in a common way.
The challenges encountered in this study highlight the impracticality of adding data collection to practice when the existing health care system does not routinely use electronic health records and time is not routinely allocated for significant documentation of patient care. In the future, the changes of using the NCP and the NCPT should all be fully implemented and electronic health record or existing registry systems should already have the data entry expected as part of routine care before attempting to conduct a research study in practice. Strategies to avoid intervention dropout for dietitians include a phased implementation as well as financial support directly to dietitians who are providing care as well as the investigators to travel to sites to resolve issues involved with implementing guideline care. However, it should be noted that once dietitians are paid to participate in research, it may not be truly “practice-based” research.
Future research studies that involve implementing evidence-based practice guidelines in practice may benefit from a more full integration and evaluation of the 6-step Ottawa Model for Knowledge Transfer, which includes a thorough assessment of the evidence-based innovation itself (development process and innovation attributes), potential adopters (their awareness, attitudes, knowledge/skill, concerns, and current practice), and the practice environment (patients, culture/social, structural, economic, and uncontrolled events). Following the assessment, a tailored implementation strategy addresses barrier management, transfer, and follow-up may promote less dietitian dropout in future studies. Throughout the research study, the adoption should be monitored with regard to intention to implement as well as actual use of EBNPGs using one of the tools specifically addressed for the purpose. If the focus of the research is on the “process” of implementation, the results can be used to ensure fidelity of the implementation of the EBNPGs and then finally measure the outcomes relative to the patient, practitioner, and system.
Issues such as electricity infrastructure for consistent internet connection are unlikely to be resolved; however, in the future there may be unique ways to explore providing alternative power sources, for example, batteries or uninterruptable power sources, to preclude electrical outages and fluctuations.
CONCLUSION
Patients seen by dietitians in both the EBNPG and UC groups achieved significant improvements in HbA1C; however, only the EBNPG group was also able to achieve significant improvements in other parameters at 6- and 12-month follow-up. The only significant difference between groups was in the reduction of triglycerides. Despite the extensive screening process and selection criteria for study dietitians, there was significant dropout and there were barriers that prevented implementing the EBNPGs. Data were insufficient to document fidelity of the EBNPG intervention in most patients in the EBNPG group. The small amount of data available at 12 months compromised the ability to truly test the hypothesis of whether there was a difference in patient outcomes when nutrition care was provided following EBNPGs. However, despite the small sample size, patients were significantly more likely to meet LDL cholesterol, HDL cholesterol, and triglyceride goals if they were treated in the EBNPG group compared with receiving UC. This research serves to identify the future direction and types of activities that will be needed to develop/adapt and promote EBNPG use throughout India. In addition, Evidence-Based Nutrition Practice Guidelines will continue to be updated and more current versions also need to be tested. For example, there is now an updated 2015 EBNPG for Diabetes 1 and 2; however, the 2011 edition of the toolkit used in this research is still the most updated toolkit available. Although these results only reflect India, future practice-based research studies in other countries would benefit from systematically assessing, managing, and measuring the barriers and supports, monitoring the intervention and degree of use, as well as evaluating outcomes.
Footnotes
The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Funding for this project was provided by the Academy of Nutrition and Dietetics Foundation through support from Abbott International-India.
We thank the following dietitians and institutions who provided baseline and follow-up data for this study: (1) usual care group: Ms Sherin Varghese (Malabar Institute of Medical Sciences, Calicut, Kerala, India), Ms Remya Paul Mukkathu (Caritas Hospital, Kottayam, Kerala, India), Ms Padma Pinnamaneni (Star Hospitals, Hyderabad, Andhra Pradesh, India), Ms Arati Borah (Barman Diabetes Specialities, Guwahati, Assam, India), Ms Kiran Dalal (Fortis Escorts Hospital, Faridabad, Haryana, India), Ms Smitha Shah (Gujarat Endocrine Centre, Dia Care Amdavad, Gujarat, India), Ms Medhavi Gautham (Santokba Durlabji Memorial Hospital, Jaipur, Rajasthan, India), Ms Yamini Atri (Metro Heart Institute with Multispecialty, Faridabad, Haryana, India), and Dr Geeta Dharmatti (Aditya Birla Memorial Hospital, Pune, Maharashtra, India); and (2) evidence-based nutrition practice guideline group: Ms Bamini Murgesh (Sundaram Medical Foundation, Chennai, Tamil Nadu, India), Ms Kavita V (PSG Hospitals, Coimbatore, Tamil Nadu, India), Ms Hema Devi Doddapaneni (Nagajuna Hospital, Vijayawada, Andhra Pradesh, India), Ms Anuradha Borah (Barman Diabetes Specialities, Guwahati, Assam, India), Ms Shilpa Thakur (Asian Institute of Medical Sciences, Faridabad, Haryana, India), Ms Charu Dua (Pushpanjali Crosslay Hospital, National Capital Region, Uttar Pradesh, India), and Ms Vaidehi Amogh Nawathe (Bhakti Vedanta Hospital, Mumbai, Maharashtra, India). Dr Vinita Satavrat, Head Medical-ANI (Abbott International-India) and her staff provided invaluable support during the project by processing central ethics review and supporting the equipment, supplies, and training on blood glucose monitoring equipment.
REFERENCES
- 1.National Academy of Sciences. Preparing for the 21st Century: Focusing on Quality in a Changing Healthcare System. Washington, DC: National Academy of Sciences Press; 1997. [Google Scholar]
- 2.Franz MJ, Horton ES, Sr, Bantle JP, et al. Nutrition principles for the management of diabetes and related complications. Diabetes Care. 1994;17(5):490–518. [DOI] [PubMed] [Google Scholar]
- 3.Harris MI, Eastman RC, Siebert C. The DCCT and medical care for diabetes in the U.S. Diabetes Care. 1994;17(7):761–764. [DOI] [PubMed] [Google Scholar]
- 4.Sudsawad P. Knowledge translation: introduction to models, strategies, and measures. http://www.ncddr.org/kt/products/ktintro/. Published 2007. Accessed November 19, 2015.
- 5.National Collaborating Centre for Method and Tools. Ottawa Model of Research Use: a framework for adopting innovations. http://www.nccmt.ca/resources/search/65. Published 2010. Accessed November 21, 2015.
- 6.Damschroder LJ, Aron DC, Keith RD, Kirsh ST, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellen M. Knowledge translation framework for ageing and health. http://www.who.int/ageing/publications/knowledge_translation.pdf?ua=1. Published 2012. Accessed November 19, 2015.
- 8.Graham ID, Logan J, Harrison MB, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006;26(1):13–24. [DOI] [PubMed] [Google Scholar]
- 9.Graham ID, Logan J. Innovations in knowledge transfer and continuity of care. Can J Nurs Res. 2004;32(4):89–103. [PubMed] [Google Scholar]
- 10.Cho NH, Whiting D, Forouhi N, et al. 4.6 South East Asia Regional Fact Sheet. In: IDF Diabetes Atlas—7th Edition. http://www.diabetesatlas.org/. Published 2015. Accessed October 31, 2016.
- 11.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. [DOI] [PubMed] [Google Scholar]
- 12.Chandalia M, Abate N, Garg A, Stray-Gundersen J, Grundy SM. Relationship between generalized and upper body obesity to insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84(7):2329–2335. [DOI] [PubMed] [Google Scholar]
- 13.Sicree R, Shaw J, Zimmett P. Diabetes and impaired glucose tolerance. Diabetes Atlas. 2006; http://www.eatlas.idf.org. Accessed November 19, 2015.
- 14.World Bank; World development indicators. http://databank.worldbank.org/data/reports.aspx?source=2&country=IND&series=&period=. Published 2014. Accessed November 19, 2015. [Google Scholar]
- 15.Raji A, Seely EW, Arky RA, Simonson DC. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab. 2001;86(11):5366–5371. [DOI] [PubMed] [Google Scholar]
- 16.Pandey A, Chawla S, Guchhait P. Type-2 diabetes: current understanding and future perspectives. IUBMB Life. 2015;67(7):506–513. [DOI] [PubMed] [Google Scholar]
- 17.Yajnik CS, Ganpule-Rao AV. The Obesity-Diabetes Association: what is different in Indians? Int J Low Extrem Wounds. 2010;9(3):113–115. [DOI] [PubMed] [Google Scholar]
- 18.Pratyush DD, Tiwari S, Singh S, Singh SK. Risk factors of diabetes in North Indians with metabolic syndrome. Diabetes Metab Syndr. 2016;10(2)(suppl 1):S68–S71. [DOI] [PubMed] [Google Scholar]
- 19.Gulati S, Misra A. Sugar intake, obesity, and diabetes in India. Nutrients. 2014;6(12):5955–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gokulakrishnan K, Deepa M, Monickaraj F, Mohan V. Relationship of body fat with insulin resistance and cardiometabolic risk factors among normal glucose-tolerant subjects. J Postgrad Med. 2011;57(3):184–188. [DOI] [PubMed] [Google Scholar]
- 21.Bantle JP, Wylie-Rosett J, Albright AL, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(suppl 1):S61–S78. [DOI] [PubMed] [Google Scholar]
- 22.Franz MJ, Monk A, Barry B, et al. Effectiveness of medical nutrition therapy provided by dietitians in the management of non–insulin-dependent diabetes mellitus: a randomized, controlled clinical trial. J Am Diet Assoc. 1995;95(9):1009–1017. [DOI] [PubMed] [Google Scholar]
- 23.Wolf AM, Conaway MR, Crowther JQ, et al. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27(7):1570–1576. [DOI] [PubMed] [Google Scholar]
- 24.Lemon CC, Lacey K, Lohse B, Hubacher DO, Klawitter B, Palta M. Outcomes monitoring of health, behavior, and quality of life after nutrition intervention in adults with type 2 diabetes. J Am Diet Assoc. 2004;104(12):1805–1815. [DOI] [PubMed] [Google Scholar]
- 25.Kulkarni K, Castle G, Gregory R, et al. Nutrition practice guidelines for type 1 diabetes mellitus positively affect dietitian practices and patient outcomes. The Diabetes Care and Education Dietetic Practice Group. J Am Diet Assoc. 1998;98(1):62–70; quiz 71–72. [DOI] [PubMed] [Google Scholar]
- 26.Standards of Medical Care in Diabetes—2016: Summary of revisions. Diabetes Care. 2016;39(suppl 1):S4–S5. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Medicare & Medicaid Services. Decision Memo for Medical Nutrition Therapy Benefit for Diabetes & ESRD (CAG-00097N). Washington, DC: Centers for Medicare & Medicaid Services; 2002. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=53&fromdb=true. Accessed January 9, 2017. [Google Scholar]
- 28.Centers for Medicare & Medicaid Services. Code of Federal Regulations, Title 42, Chapter IV, Subchapter B, Part 410, Subpart G, §410.130 Definitions. Washington, DC: Office of the Federal Register and the Government Publishing Office; 2013. https://ecfr.io/Title-42/pt42.2.410#se42.2.410_1130. Accessed January 9, 2017. [Google Scholar]
- 29.Wylie-Rosett J, Cypress M, Walker E, Engel S, D'Eramo-Melkus G, DiLorenzo T. Assessment of nutrition care provided to patients with diabetes in primary-care clinics. J Am Diet Assoc. 1992;92(7):854–856. [PubMed] [Google Scholar]
- 30.Anderson EJ, Richardson M, Castle G, et al. Nutrition interventions for intensive therapy in the diabetes control and complications trial. The DCCT Research Group. J Am Diet Assoc. 1993;93(7):768–772. [DOI] [PubMed] [Google Scholar]
- 31.American Dietetic Association. ADA MNT Evidence-Based Guides for Practice. Nutrition Practice Guidelines for Type 1 and 2 Diabetes (book on CD-ROM). Chicago, IL: American Dietetic Association; 2001. [Google Scholar]
- 32.Academy of Nutrition and Dietetics. Evidence-Based Nutrition Practice Guidelines—Diabetes (DM) Type 1 and 2 (2006-2007). http://www.andeal.org/. Published 2006. Accessed November 19, 2015.
- 33.Academy of Nutrition and Dietetics. Diabetes Mellitus Type (DM) 1 and 2 Evidence-Based Nutrition Practice Guideline. http://www.andeal.org. Published 2008. Accessed November 19, 2015.
- 34.Haase F, Swan W, Wedel N. Diabetes Mellitus Toolkit. Chicago, IL: Academy of Nutrition and Dietetics; 2011. [Google Scholar]
- 35.Diabetes (DM) Guideline (2015). Evidence Analysis Library. https://www.andeal.org/. Published 2015. Accessed November 1, 2016.
- 36.Diabetes (DM) Type 1 and 2 (2013-2015). Evidence Analysis Library. https://www.andeal.org/. Published 2013. Accessed November 1, 2016.
- 37.Diabetes (DM) Guideline (2008). Evidence Analysis Library. https://www.andeal.org/. Published 2008. Accessed November 1, 2016.
- 38.Academy of Nutrition and Dietetics. International Dietetics & Nutrition Terminology (IDNT) Reference Manual: Standardized Language for the Nutrition Care Process. 4th ed. Chicago, IL: Academy of Nutrition and Dietetics; 2013. [Google Scholar]
- 39.Trostler N, Myers E, Alphan E, Endevelt R. Outcomes monitoring and implementing evidence-based nutrition practice guidelines for type 2 diabetes mellitus in 2 Middle Eastern countries. Top Clin Nutr. 2013;28(3):233–248. [Google Scholar]
- 40.Academy of Nutrition and Dietetics. Diabetes 1 and 2: Executive Summary of Recommendations. http://www.andeal.org/topic.cfm?menu=3251&cat=3252. Published 2008. Accessed December 18, 2015.