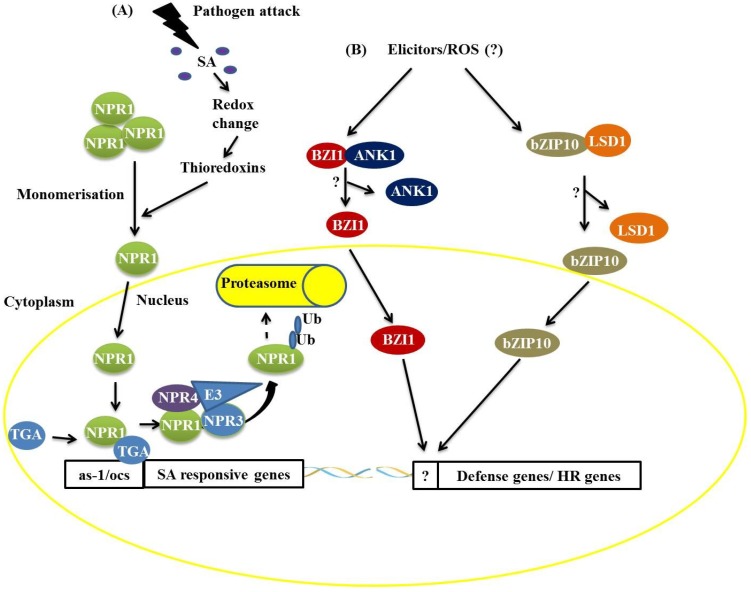
Figure 1.
Two distinct mechanisms of basic leucine zipper containing domain proteins (bZIP) protein actions during plant defense responses. (A) The attack of a biotrophic pathogen triggers a signaling pathway mediated by salicylic acid resulting in the dissociation of the non-expresser of pathogen-related (PR) (NPR1) protein, which translocates to the nucleus and activates the expression of SA-responsive genes by interaction with the TGACGTCA cis-element-binding protein (TGA) bZIP trans-acting factors. The NPR1 protein is ubiquitinated and targeted for degradation by the 26S proteasome complex; (B) Recognition of elicitors after pathogen attack promotes the dissociation of the BZI1/ANK1 and AtbZIP10/LSD1 complexes, favoring the positive transcriptional regulation of hypersensitive response (HR)- and basal defense-related genes.