Figure 3. CD3CAR NK-92 cells display robust killing ability for multiple primary CD3+ leukemic cells obtained from patient bone marrow aspirate samples.
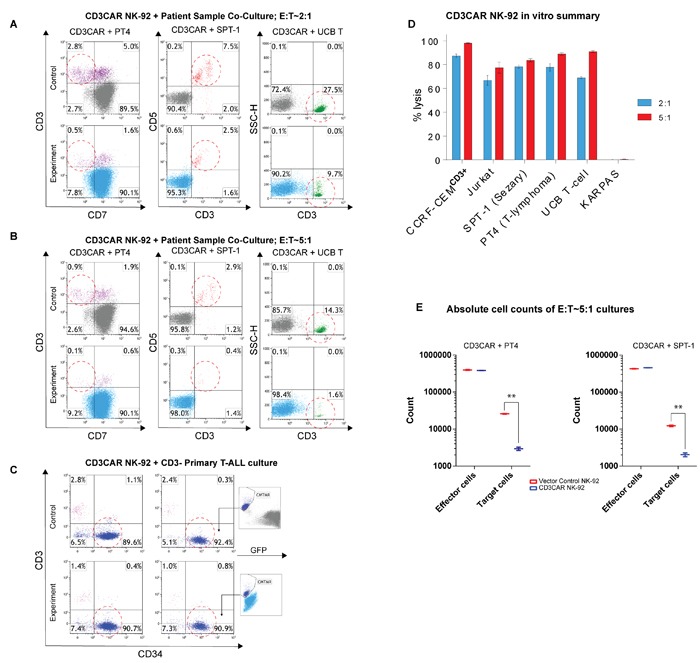
A. Co-cultures were carried out at an E:T ratio of 2:1 for 24 hours. Ability of CD3CAR NK-92 cells to lyse target cells, % cell lysis, determined by relative amounts of residual target cells post co-culture. CD3CAR NK-92 cells lyse patient sample PT4 (N = 4) (unclassified PTCL phenotype CD7- CD3+) and SPT-1 (Sézary Syndrome) (N = 2) leukemic cells obtained from patient bone marrow aspirate expressing CD3 as well as normal human T cells (UCB T) isolated from cord blood (N = 4). B. Co-cultures carried out for 24 hours at an E:T ratio of 5:1. Experimental conditions identical to above. C. CD3CAR NK-92 cells co-cultured with T-ALL patient sample expressing majority CD3- tumor burden with a small population of normal T-cells (purple). Co-cultures were conducted in E:T ratios of 2:1 and 5:1 for 24 hours. Cytotracker dye (CMTMR) was used to stain T-ALL sample due to heterogeneity in flow phenotype. D. Bar graphs summarizing the cytotoxic activity of CD3CAR NK-92 cells against all types of CD3+ cell populations. T-lymphoblast cell lines and patient samples from T-cell lymphomas expressing CD3 co-cultured with CD3CAR NK-92 cells in the indicated E:T (effector:target) cell ratios of 2:1 and 5:1. % cell lysis values determined using the CD3+ total population as the target population with the exception of PT4, where the CD3+ CD7- population was designated as the target cell population for enhanced specificity. E. Absolute cell counts of selected PT4 and SPT-1 co-cultures at an E:T ratio of 5:1 over 24 hours. Control and CD3CAR treatment samples are labeled in red and blue respectively. Effector and target cell counts were obtained from Kaluza flow cytometry software (Beckman Coulter).
