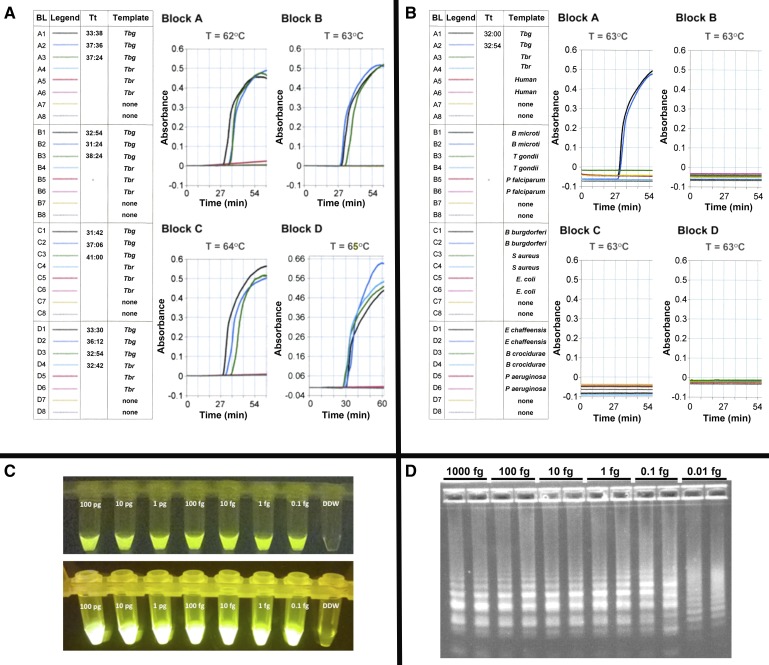
Figure 2.
Optimal reaction temperature, analytical specificity, and analytical sensitivity of TBG4 LAMP. (A) Temperature optimization. Real time TBG4 LAMP was conducted at four different temperatures (62–65°C) and 0.01 pg Trypanosoma brucei gambiense DAL 972 (Tbg) and Trypanosoma brucei rhodesiense LouTat 1A (Tbr) genomic DNA as template. LAMP in the absence of DNA templates served as controls. The data are plotted as reaction time vs. absorbance. The measurement result Tt, represents the time any sample shown in the measurement block (BL) equals or exceeds the threshold (i.e., 0.1 absorbance unit). Our data show that 0.01 pg Tbg DNA can be amplified within a time range 31–41 minutes when reactions are conducted at 62–65°C. However, a faster reaction time (31′24″) was observed at 63°C, hence its selection for further experiments. The slight time differences for the LAMP positive wells are not significant. Note that specificity for Tbg DNA diminished with assay temperatures ≥ 65°C. (B) Analytical Specificity. Real time LAMP was conducted at 63°C (60 minutes) using 0.01 pg genomic DNA from T. b. gambiense DAL 972 (Tbg), T. b. rhodesiense LouTat 1A (Tbr), human, Babesia microti, Toxoplasma gondii, Plasmodium falciparum, Borrelia burgdorferi, Staphylococcus aureus, Ehrlichia Chaffeensis, and Pseudomonas aeruginosa as template. LAMP in the absence of DNA templates served as controls. Analytical sensitivity. TBG4 LAMP was run at 63°C (60 minutes) using 1:10 serial dilutions of T. b. gambiense DAL 972 and 348BT DNA. (C) Shows the detection of LAMP amplified T. b. gambiense 348BT DNA in reaction tubes containing calcein (Eiken Chemical Co., Tochigi, Japan) as seen on (upper panel) a black background with normal lighting and (lower panel) under a BLOOK LED transilluminator (Gene Direx, Taqkey Science Co., Ltd., Miaoli County, Taiwan) are shown. In both cases, TBG4 LAMP was able to detect 0.1 fg of parasite DNA. (D) The detection of LAMP-amplified T. b. gambiense DAL 972 DNA by agarose gel electrophoresis. Generally, there is no specific band pattern observed in LAMP gel electrophoresis, however, in this study we observed that the density of amplified fragments is greater (∼6- to 7-fold) at higher concentrations (1,000–1 fg) and are reduced (∼4–5) at lower concentrations (0.1–0.01 fg). Although 0.01 fg DNA could be detected, the altered electrophoretic banding pattern using 0.01 fg DNA prompts us to conservatively set the sensitivity limit to be at least 0.1 fg DNA. LAMP = loop-mediated isothermal amplification.