Abstract
Red cell distribution width (RDW) is linked to cardiovascular risk in the general population, an association that might be driven by inflammation. Whether this relationship holds for patients with Human Immunodeficiency Virus (HIV) infection has not been previously studied. Using a large clinical registry, we show that elevated RDW (>14.5%) is independently associated with increased risk of coronary artery disease (odds ratio (OR) 1.39 [1.25–1.55]), peripheral vascular disease (OR 1.41 [1.29–1.53]), myocardial infarction (1.43 [1.25–1.63]), heart failure (OR 2.23 [1.99–2.49]), and atrial fibrillation (OR 1.96 [1.64–2.33]). In conclusion, in the context of the inflammatory milieu that accompanies HIV infection, RDW remains a powerful marker of CV disease.
Keywords: Cardiovascular disease, coronary, peripheral, heart failure, RDW
Introduction
Red cell distribution width (RDW) is a measure of the variability of red blood cell size, also known as anisocytosis. Over the past decade, RDW has also been associated with incident myocardial infarction1,2 and heart failure3 in the general population. RDW has also emerged as one of the strongest predictors of poor survival in patients with established heart failure4–6 and coronary artery disease7–10. Anisiocytosis is typically a result of impaired iron metabolism11 and tends to correlate with low hemoglobin, reduced mean corpuscular volume, high iron binding capacity, and elevated erythropoietin levels despite normal ferritin levels. RDW has also been proposed as marker of immune activation, correlating with levels of tumor necrosis factor (TNF)-α12 and interleukin (IL)- 611. The processes of inflammation and dysregulated hematopoiesis may be linked, since IL-6 is crucial for the production of hepcidin in the liver, and thus may indirectly regulate iron metabolism.
Antiretroviral therapy has improved life expectancy in patients living with HIV, but the burden cardiovascular disease in this population is increasingly recognized13. Patients with HIV have a high prevalence of silent and clinically apparent cardiovascular diseases14–16. We and others have shown that cardiovascular disease in patients with HIV seems to be associated with inflammation, including IL-617–21 elevations, but as intra-individual variability in IL-6 levels is high, the identification of “at risk” patients remains problematic. The RDW is routinely reported as part of automated blood count from clinical laboratories, and therefore could be an attractive pragmatic biomarker of immune activation and cardiovascular risk. We therefore sought to test whether RDW is associated with cardiovascular disease prevalence specifically in patients with HIV.
Methods
Data source
Explorys (Explorys, Inc; Cleveland, Ohio) is a commercial cloud-based database that aggregates data from electronic health records of participating hospital systems. It currently encompasses 23 integrated health systems consisting of 360 hospitals, 315,000 providers, and about 50 million unique patients. It collects data through a healthcare gateway server behind the firewall of participating institutions. The data are collected from billing inquiries, electronic health records, and laboratory systems. These data aggregates are then de-identified and standardized into Unified Medical Language Systems (UMLS) ontologies to facilitate searching and indexing22. Diagnoses are mapped into systematized nomenclature of medicine-clinical terms (SNOMED-CT) hierarchy, prescriptions mapped to RxNorm, and laboratory test observations mapped to logistical observation identifier names and codes (LOINC). Data collection started in 1999 and are updated every 24–48 hours. The platform is compliant with the Health Insurance Portability and Accountability Act (HIPAA) and Health Information Technology for Economic and Clinical Health (HITECH) Act standards; hence, its use is exempted from Institutional Review Board review under a pre-specified policy. The database rounds number of patients to the nearest 10 for an added data protection. This platform has been used for research purposes and has been validated in fields of oncology23, orthopedics24, gastroenterology25, gynecology26 among others.
Cohort selection and definitions
We selected all patients who are at least 18 years of age with a diagnosis of “Human Immunodeficiency Virus Infection” who had at least one measurement of RDW after documentation of HIV infection. High RDW (>14.5%) and low/normal RDW (</=14.5%) were defined in accordance with previously used cut-offs27,28. Cardiovascular diseases were identified by their umbrella terms: “disorder of coronary artery”, “peripheral vascular disease”, “heart failure”, “myocardial infarction” and “atrial fibrillation”. We calculated the prevalence of cardiovascular diseases separately in patients (18–65 years old) with or without HIV using their most recent RDW. This analysis was also replicated in patients without anemia (hemoglobin >12 g/dL) to test the effect of RDW in non-anemic patients, and in Caucasians to evade the possible interaction between RDW and sickle-cell trait that is prevalent in African Americans.
We also selected a cohort with HIV without CVD and then followed for incident cardiovascular diseases (myocardial infarction or heart failure) stratified by the first RDW after documentation of HIV infection. This analysis was adjusted for traditional cardiovascular risk factors: age, gender, hypertension, diabetes, and dyslipidemia.
Statistical analysis
The data are presented as numbers and percentages. Pearson’s chi-squared test was used to test for differences. Logistic regression models were used to identify the adjusted odds ratio of cardiovascular events. Social Package for Social Studies (SPSS) version 19.0 was used for all analyses, with significance level set at p<0.05.
Results
Of the 30,590,990 adults (18–65 years) in the database at the time of the inquiry, 79,590 (0.26%) had HIV infection and 46,720 had at least one documented RDW. Of those who were HIV-infected, 16,570 patients (35% of the total HIV population) had an elevated RDW. Patients with HIV and a high RDW had several important clinical and demographic differences compared to those with a low or normal RDW (Table 1). High RDW patients were slightly older, more likely to be female (39% vs 26%, p<0.001), African American (62% vs 42%, p<0.001), and to have diabetes (22% vs 13%, p<0.001), hypertension (48% vs 36%, p<0.001), and to be actively smoking (45% vs 40%, p<0.001), compared to individuals with low or normal RDW. HIV-infected patients with elevated RDW were also less likely to be treated with antiretroviral therapy, compared to HIV-infected patients with a low/normal RDW (50% vs. 53%, p<0.001). Differences in subclasses of ART use are displayed in Table 1.
Table 1.
Patient characteristics stratified by HIV status and RDW category. P-value <0.001 for all comparisons between RDW categories.
| HIV+ | HIV− | |||
|---|---|---|---|---|
| Low RDW | High RDW | Low RDW | High RDW | |
| Number of patients | 30,150 | 16,570 | 7,969,250 | 1,617,900 |
| Age (years) | ||||
| 20–24 | 800 (3%) | 270 (2%) | 598,560 (8%) | 78,630 (5%) |
| 25–29 | 2,140 (7%) | 730 (4%) | 802,550 (10%) | 118,250 (7%) |
| 30–34 | 2,570 (9%) | 1,050 (6%) | 873,500 (11%) | 141,010 (9%) |
| 35–39 | 3,100 (10%) | 1500 (9%) | 864,190 (11%) | 153,850 (10%) |
| 40–44 | 3,390 (11%) | 1,990 (12%) | 810,060 (10%) | 159,670 (10%) |
| 45–49 | 4,760 (16%) | 2,830 (17%) | 865,350 (11%) | 184,790 (11%) |
| 50–54 | 5,720 (19%) | 3,200 (19%) | 925,350 (12%) | 213,800 (13%) |
| 55–59 | 4,340 (14%) | 2,740 (17%) | 984,120 (12%) | 242,890 (15%) |
| 60–64 | 2,860 (9%) | 1,930 (12%) | 910,420 (11%) | 255,090 (16%) |
| Gender | ||||
| Male | 22,320 (74%) | 10,110 (61%) | 3,419,430 (43%) | 496,580 (31%) |
| Female | 7,830 (26%) | 6,450 (39%) | 4,547,640 (57%) | 1,120,910 (69%) |
| Race | ||||
| African American | 12,740 (42%) | 10,200 (62%) | 911,050 (11%) | 427,450 (26%) |
| Caucasian | 14,450 (48%) | 5,090 (31%) | 5,859,390 (74%) | 966,950 (60%) |
| Insurance | ||||
| Private | 13,670 (45%) | 5,750 (35%) | 4,516,050 (57%) | 811,620 (50%) |
| Medicaid | 7,420 (25%) | 5,010 (30%) | 910,970 (11%) | 279,160 (17%) |
| Medicare | 5,750 (19%) | 4,130 (25%) | 503,640 (6%) | 199,900 (12%) |
| Antiretroviral therapy | ||||
| Any ART | 15,990 (53%) | 8,280 (50%) | N/A | N/A |
| Protease Inhibitor | 8,600 (29%) | 5,530 (33%) | N/A | N/A |
| Integrase Inhibitor | 2,460 (8%) | 1,550 (9%) | N/A | N/A |
| Reverse Transcriptase Inhibitor | 15,390 (51%) | 7,900 (48%) | N/A | N/A |
| Tenofovir | 12,600 (42%) | 5,700 (34%) | N/A | N/A |
| Thymidine analogue (Stavudine/Zidovudine) | 2,600 (9%) | 2,080 (13%) | N/A | N/A |
| Risk factors | ||||
| Diabetes | 4,030 (13%) | 3,630 (22%) | 684,700 (9%) | 288,620 (18%) |
| Hypertension | 10,740 (36%) | 7,990 (48%) | 1,853,780 (23%) | 603,720 (37%) |
| Dyslipidemia | 9,180 (30%) | 4,540 (27%) | 1,747,490 (22%) | 413,160 (26%) |
| Smoking | 11,970 (40%) | 7,380 (45%) | 1,383,800 (17%) | 361,270 (22%) |
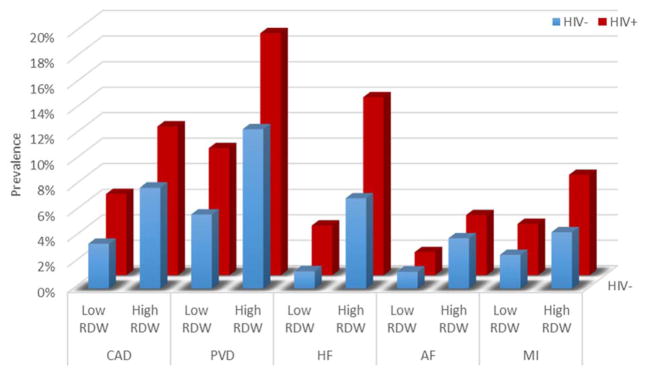
In the HIV negative population, an elevated RDW was associated with a higher prevalence of cardiovascular diseases, compared to those with a low/normal RDW (Figure 1). For instance, among the uninfected, patients with a high RDW had higher prevalence of coronary artery disease (8% vs 3%, p<0.001) and heart failure (7% vs 1%, p<0.001), compared with those having a normal or low RDW. HIV was associated with a higher prevalence of cardiovascular disease compared to HIV uninfected subjects, regardless of RDW status. For example, among patients with high RDW, CAD (12% vs 8%, p<0.001) and HF (14% vs 7%, p<0.001) were more prevalent in HIV+ than uninfected controls. Patients with HIV were more likely to have an elevated RDW compared to the HIV uninfected (35% vs. 17%, p<0.001).
Figure 1.
Prevalence of CVD in patients with or without HIV stratified by RDW category. P-value <0.001 for all comparisons between high vs. low RDW categories for both HIV+ and HIV− patients.
Patients with HIV infection and an elevated RDW had a higher prevalence of cardiovascular disease compared to HIV+ subjects with a low/normal RDW. For instance, HIV+ patients with high RDW had higher prevalence of coronary artery disease (12% vs 6%, p<0.001), prior myocardial infarction (8% vs 4%, p<0.001), and peripheral vascular disease (19% vs 10%, p<0.001). Patients with HIV and an elevated RDW also had a higher prevalence of heart failure compared to low/normal RDW HIV+ patients (14% vs 4%, p<0.001). Atrial fibrillation also tended to be more common in patients with elevated RDW among patients living with HIV (5% vs 2%, p<0.001).
To determine whether anemia or hemoglobinopathies might be confounding the association between RDW and CVD, two sensitivity analyses were performed. In HIV+ patients without anemia, the relationships between RDW and CVD remained unchanged: CAD (high RDW 10% vs low/normal 6%, p<0.001), PVD (high RDW 16% vs low/normal 10%, p<0.001), HF (high RDW 9% vs low/normal 3%, p<0.001), MI (high RDW 7% vs low/normal 4%, p<0.001) and AF (high RDW 4% vs low/normal 2%, p<0.001). Similar findings persisted in the Caucasian subgroup: CAD (high RDW 11% vs low/normal 7%, p<0.001), PVD (high RDW 18% vs low/normal 10%, p<0.001), HF (high RDW 11% vs low/normal 3%, p<0.001), MI (high RDW 9% vs low/normal 4%, p<0.001) and AF (high RDW 5% vs low/normal 2%, p<0.001).
An elevated RDW in HIV patients without CVD was associated with the development of future disease, even after adjustment for age, gender, hypertension, diabetes, and dyslipidemia. Compared to low/normal RDW HIV+ patients, those HIV+ patients with an elevated RDW had increased odds of incident coronary artery disease (unadjusted OR [95% CI] 1.42 [1.29–1.56], p<0.001); adjusted OR 1.39 [1.25–1.55], p<0.001), peripheral vascular disease (unadjusted OR 1.49 [1.38–1.61], p<0.001; adjusted OR 1.41 [1.29–1.53], p<0.001), myocardial infarction (unadjusted OR 1.43 [1.26–1.62], p<0.001; adjusted 1.43 [1.25–1.63], p<0.001), heart failure (unadjusted OR 2.39 [2.16–2.65], p<0.001; adjusted OR 2.23 [1.99–2.49], p<0.001), and atrial fibrillation (unadjusted OR 2.04 [1.72–2.41], p<0.001; adjusted OR 1.96 [1.64–2.33], p<0.001).
Discussion
In this large electronic medical record cohort, we find that irrespective of HIV status, an elevated RDW is associated with a higher burden of atherosclerotic cardiovascular disease, heart failure, and atrial fibrillation. Thus, despite differences in the inflammatory milieu and medication exposures between those with and without HIV, the mechanisms that link RDW and cardiovascular disease appear to remain relevant. Given the availability of RDW during routine clinical practice and its strong relationship to CVD, these findings suggest additional study of this parameter as a possible immune and cardiovascular biomarker in HIV are warranted.
Several studies have described the association between RDW and cardiovascular disease in the general population. Felker et al29 were the first to demonstrate the association of RDW and heart failure outcomes in the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) program (n=2,679) and Duke Database (n=2,140). This association was independent of other laboratory and clinical predictors including age and was more powerful than functional class or ejection fraction. Other similar studies have confirmed this relationship30. In a population-based analysis of 25,612 persons in Norway, there was a linear association between baseline RDW and risk of myocardial infarction, with 13% increase in MI risk for every 1% increment in RDW31, which persisted after exclusion of patients with anemia32. Similarly, studies have associated RDW with the presence of coronary artery disease33, peripheral vascular disease34, and atrial fibrillation35. We are unaware of any prior report describing the relationship between RDW and cardiovascular disease in the context of HIV infection. Thus, our study suggests that the relationship between RDW and CVD holds in HIV infection. This is particularly interesting as both immunologic and erythropoetic perturbations are characteristic of HIV disease and its treatment.
There are a number of factors that may explain the RDW-CV disease association. Increased RDW can be observed in states of ineffective red blood cell production, including vitamin deficiency, malnutrition states, and anemia of chronic disease36. How such bone marrow dysfunction might promote cardiovascular disease is uncertain. Several groups have ascribed the relationship between RDW and cardiovascular disease to subclinical immune activation, which has been previously linked to cardiovascular disease. For example, RDW has been associated with elevated levels of C-reactive protein, tumor necrosis factor alpha12, malondialdehyde12, and interleukin-637, all of which have been associated with CV disease. Inflammatory cytokines can worsen red blood cell survival, lead to erythropoietin resistance, and stimulate the production of hepcidin. Each of these mechanisms may impair red blood cell production and increase RDW.
A previous study by Gallegio et al did not find an association between RDW and the aggregate of traditional cardiovascular risk factors (Framingham risk score) in patients with HIV38. In our current study, an elevated RDW was associated with modest differences in some, but not all, cardiovascular risk factors. We therefore sought to examine whether the association between RDW and CVD was linked to traditional risk factors. After adjustment for age, gender, dyslipidemia, diabetes, and hypertension, an elevated RDW independently predicts cardiovascular events. Thus, RDW may have added value to improve cardiovascular risk assessment in addition to traditional risk factors in patients with HIV. Future studies are needed to explore the extent to which RDW correlates with immune activation, which has also been linked to cardiovascular disease in HIV, and whether RDW might also be a marker of immunologic outcomes in HIV.
This large analysis is limited by its retrospective design, lack of patient-level data, and the potential variability of RDW measurements across different hospital systems. We were unable to explore in any detail the mechanisms of the observed associations. Because of these limitations, this study must be interpreted with caution as hypothesis generating. Nevertheless, the substantial risk differences and large sample size provide a broad observational overview of these relationships in a real-world population.
Conclusion
In patients with HIV, an elevated RDW is associated with a higher risk of cardiovascular disease. The connections between bone marrow function, immune activation, and thrombosis should be examined further. If validated prospectively, the RDW may be a convenient, pragmatic biomarker of risk in addition to that conferred by HIV and traditional risk factors alone.
Acknowledgments
Funding
This work was funded by the Clinical and Translational Science Collaborative of Cleveland, KL2TR000440 to DAZ, from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research; and by K23 HL123341 to CTL. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zalawadiya SK, Veeranna V, Niraj A, Pradhan J, Afonso L. Red cell distribution width and risk of coronary heart disease events. Am J Cardiol. 2010 Oct 1;106(7):988–993. doi: 10.1016/j.amjcard.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Wang P, Wang Y, Li H, Wu Y, Chen H. Relationship between the red blood cell distribution width and risk of acute myocardial infarction. J Atheroscler Thromb. 2015;22(1):21–26. doi: 10.5551/jat.23937. [DOI] [PubMed] [Google Scholar]
- 3.Borne Y, Smith JG, Melander O, Hedblad B, Engstrom G. Red cell distribution width and risk for first hospitalization due to heart failure: a population-based cohort study. Eur J Heart Fail. 2011 Dec;13(12):1355–1361. doi: 10.1093/eurjhf/hfr127. [DOI] [PubMed] [Google Scholar]
- 4.Felker GM, Allen LA, Pocock SJ, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007 Jul 3;50(1):40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 5.Al-Najjar Y, Goode KM, Zhang J, Cleland JG, Clark AL. Red cell distribution width: an inexpensive and powerful prognostic marker in heart failure. Eur J Heart Fail. 2009 Dec;11(12):1155–1162. doi: 10.1093/eurjhf/hfp147. [DOI] [PubMed] [Google Scholar]
- 6.Oh J, Kang SM, Hong N, et al. Relation between red cell distribution width with echocardiographic parameters in patients with acute heart failure. J Card Fail. 2009 Aug;15(6):517–522. doi: 10.1016/j.cardfail.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Dabbah S, Hammerman H, Markiewicz W, Aronson D. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol. 2010 Feb 1;105(3):312–317. doi: 10.1016/j.amjcard.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Uyarel H, Ergelen M, Cicek G, et al. Red cell distribution width as a novel prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis. 2011 May;22(3):138–144. doi: 10.1097/MCA.0b013e328342c77b. [DOI] [PubMed] [Google Scholar]
- 9.Tziakas D, Chalikias G, Grapsa A, Gioka T, Tentes I, Konstantinides S. Red blood cell distribution width: a strong prognostic marker in cardiovascular disease: is associated with cholesterol content of erythrocyte membrane. Clin Hemorheol Microcirc. 2012;51(4):243–254. doi: 10.3233/CH-2012-1530. [DOI] [PubMed] [Google Scholar]
- 10.Gul M, Uyarel H, Ergelen M, et al. The relationship between red blood cell distribution width and the clinical outcomes in non-ST elevation myocardial infarction and unstable angina pectoris: a 3-year follow-up. Coron Artery Dis. 2012 Aug;23(5):330–336. doi: 10.1097/MCA.0b013e3283564986. [DOI] [PubMed] [Google Scholar]
- 11.Allen LA, Felker GM, Mehra MR, et al. Validation and Potential Mechanisms of Red Cell Distribution Width as a Prognostic Marker in Heart Failure. Journal of Cardiac Failure. 2010 Mar;16(3):230–238. doi: 10.1016/j.cardfail.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorente L, Martín MM, Abreu-González P, et al. Red Blood Cell Distribution Width during the First Week Is Associated with Severity and Mortality in Septic Patients. PLoS One. 2014;9(8):e105436. doi: 10.1371/journal.pone.0105436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United States: 1999 to 2013. Am J Cardiol. doi: 10.1016/j.amjcard.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currier JS, Lundgren JD, Carr A, et al. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–e35. doi: 10.1161/CIRCULATIONAHA.107.189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolan SE, Hadigan C, Killilea KM, et al. Increased cardiovascular disease risk indices in HIV-infected women. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2005;39(1):44–54. doi: 10.1097/01.qai.0000159323.59250.83. [DOI] [PubMed] [Google Scholar]
- 16.Friis-Møller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients–association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17(8):1179–1193. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 17.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, Coagulation and Cardiovascular Disease in HIV-Infected Individuals. PLoS ONE. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordell AD, McKenna M, Borges ÁH, et al. Severity of Cardiovascular Disease Outcomes Among Patients With HIV Is Related to Markers of Inflammation and Coagulation. Journal of the American Heart Association. 2014 Jun 19;3(3) doi: 10.1161/JAHA.114.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longenecker CT, Funderburg NT, Jiang Y, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013;14(6):385–390. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longenecker CT, Jiang Y, Orringer CE, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS (London, England) 2014;28(7):969. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204(8):1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaelber DC, Foster W, Gilder J, Love TE, Jain AK. Patient characteristics associated with venous thromboembolic events: a cohort study using pooled electronic health record data. J Am Med Inform Assoc. 2012 Nov-Dec;19(6):965–972. doi: 10.1136/amiajnl-2011-000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Kindi SG, Oliveira GH. Prevalence of Preexisting Cardiovascular Disease in Patients With Different Types of Cancer: The Unmet Need for Onco-Cardiology. Mayo Clin Proc. 2015 Nov 18; doi: 10.1016/j.mayocp.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Pfefferle K, Gil K, Fening S, Dilisio M. Validation study of a pooled electronic healthcare database: the effect of obesity on the revision rate of total knee arthroplasty. Eur J Orthop Surg Traumatol. 2014 Dec 01;24(8):1625–1628. doi: 10.1007/s00590-014-1423-2. [DOI] [PubMed] [Google Scholar]
- 25.Maradey-Romero C, Prakash R, Lewis S, Perzynski A, Fass R. The 2011–2014 prevalence of eosinophilic oesophagitis in the elderly amongst 10 million patients in the United States. Aliment Pharmacol Ther. 2015 Mar 23; doi: 10.1111/apt.13171. [DOI] [PubMed] [Google Scholar]
- 26.Yurteri-Kaplan LA, Mete MM, St Clair C, Iglesia CB. Practice patterns of general gynecologic surgeons versus gynecologic subspecialists for concomitant apical suspension during vaginal hysterectomy for uterovaginal prolapse. South Med J. 2015 Jan;108(1):17–22. doi: 10.14423/SMJ.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 27.Ye Z, Smith C, Kullo IJ. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. The American journal of cardiology. 2011;107(8):1241–1245. doi: 10.1016/j.amjcard.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson CE, Dalzell JR, Bezlyak V, et al. Red cell distribution width has incremental prognostic value to B-type natriuretic peptide in acute heart failure. Eur J Heart Fail. 2009;11(12):1152–1154. doi: 10.1093/eurjhf/hfp157. [DOI] [PubMed] [Google Scholar]
- 29.Felker GM, Allen LA, Pocock SJ, et al. Red Cell Distribution Width as a Novel Prognostic Marker in Heart Failure: Data From the CHARM Program and the Duke Databank. Journal of the American College of Cardiology. 2007 Jul 3;50(1):40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 30.Muhlestein JB, Lappe DL, Anderson JL, et al. Both initial red cell distribution width (RDW) and change in RDW during heart failure hospitalization are associated with length of hospital stay and 30-day outcomes. International Journal of Laboratory Hematology. 2016;38(3):328–337. doi: 10.1111/ijlh.12490. [DOI] [PubMed] [Google Scholar]
- 31.Skjelbakken T, Lappegard J, Ellingsen TS, et al. Red cell distribution width is associated with incident myocardial infarction in a general population: the Tromso Study. J Am Heart Assoc. 2014 Aug;3(4) doi: 10.1161/JAHA.114.001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skjelbakken T, Lappegård J, Ellingsen TS, et al. Red Cell Distribution Width Is Associated With Incident Myocardial Infarction in a General Population: The Tromsø Study. Journal of the American Heart Association. 2014 Aug 29;3(4) doi: 10.1161/JAHA.114.001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isik T, Uyarel H, Tanboga IH, et al. Relation of red cell distribution width with the presence, severity, and complexity of coronary artery disease. Coron Artery Dis. 2012 Jan;23(1):51–56. doi: 10.1097/MCA.0b013e32834e4f5c. [DOI] [PubMed] [Google Scholar]
- 34.Zalawadiya SK, Veeranna V, Panaich SS, Afonso L. Red cell distribution width and risk of peripheral artery disease: analysis of National Health and Nutrition Examination Survey 1999–2004. Vasc Med. 2012 Jun;17(3):155–163. doi: 10.1177/1358863X12442443. [DOI] [PubMed] [Google Scholar]
- 35.Gungor B, Ozcan KS, Erdinler I, et al. Elevated levels of RDW is associated with non-valvular atrial fibrillation. J Thromb Thrombolysis. 2014 May;37(4):404–410. doi: 10.1007/s11239-013-0957-1. [DOI] [PubMed] [Google Scholar]
- 36.Evans TC, Jehle D. The red blood cell distribution width. The Journal of Emergency Medicine. 1991 Jan 01;9:71–74. doi: 10.1016/0736-4679(91)90592-4. [DOI] [PubMed] [Google Scholar]
- 37.Semba RD, Patel KV, Ferrucci L, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women’s Health and Aging Study I. Clin Nutr. 2010 Oct;29(5):600–604. doi: 10.1016/j.clnu.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.María L. Gallego IAP-H, Rosario Palacios*, Josefa Ruiz-Morales, Enrique Nuño, Manuel Márquez, Jesús Santos. Red cell distribution width in patients with HIV infection. Open Journal of Internal Medicine. 2012;2:7–10. [Google Scholar]