Fig. 3.
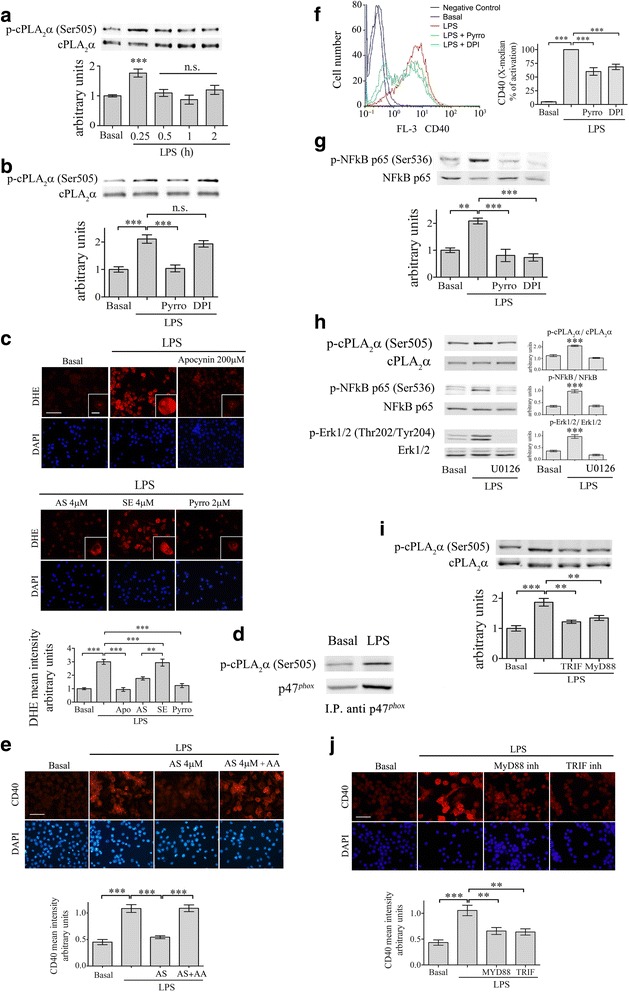
cPLA2α activates NFκB through activation of NOX2-NADPH oxidase in the BV-2 microglia cells under LPS stimulation. a A representative immunoblot analysis of the kinetics of cPLA2α phosphorylation induced by 50 ng/ml LPS, out of three independent experiments. The intensity of each phosphorylated cPLA2α (p-cPLA2α Ser-505) band after quantification by densitometry was divided by the intensity of each cPLA2α band and expressed as arbitrary units. The bar graphs are the mean ± SE from three independent experiments. b The BV-2 cells were treated with 2 μm pyrrophenone (Pyrro) or 5 μm DPI for 60 min before stimulation with 50 ng/ml LPS for 15 min. The intensity of phosphorylated cPLA2α was quantitated by densitometry as described in (a). The bar graphs are the mean ± SE from three independent experiments. c The effect of cPLA2α inhibition on superoxide production in the unstimulated or stimulated BV-2 cells with 50 ng/ml LPS for 15 min was detected by DHE reduction. Two micrometer pyropheonoe (Pyrro) or 200 μm apocynin (used as a positive control) were added to the cells 60 min before stimulation with LPS. AS or sense were added 24 h prior to addition of LPS. DAPI staining shows cell nuclei. Scale bars large = 50 μm, insert = 20 μm. The intensity of reduced DHE was quantitated and expressed in the bar graph as arbitrary units. The bar graphs are the mean ± SE from three independent experiments. d Immunoprecipitation of p47phox and phoshpo cPLA2α (pcPLA2α) in the membrane fraction of unstimulated microglia and stimulated with LPS for 15 min. Shown a representative immunoblot of three experiments. e Addition of 10 μM arachidonic acid together with LPS to cells pretreated for 24 h with antisense against cPLA2α restored the expression of CD40 protein. Shown a representative immunofluorescence staining of CD40. DAPI staining shows cell nuclei. The intensity of CD40 was quantitated for the cell and expressed in the bar graph as arbitrary units. Scale bars = 50 μm. The bar graph is the mean ± SE from three independent experiments. (***p < 0.0001). f FACS analysis of CD40 protein expression in the unstimulated or stimulated BV-2 cells with 50 ng/ml LPS for 24 h in the absence or presence of 2 μm pyrrophenone or 5 μm DPI (added to the cells 60 min before stimulation with LPS). The bar graphs are the X-median ± SE from five independent experiments. g A representative immunoblot analysis of NF-κB p-65 phosphorylation (p-NFκB p-65 Ser-536) in unstimulated or stimulated BV-2 microglia by 50 ng/ml LPS for 15 min in the absence or presence of 2 μm pyrrophenone or 5 μm DPI. The intensity of each phosphorylated NF-κB p-65 band after quantification by densitometry was divided by the intensity of each NFκB p-65 band and expressed as arbitrary units. The bar graphs are the mean ± SE from three independent experiments. h A representative immunoblot analysis of phoshpo cPLA2α and phospho NF-κB p-65 subunit and phosspho ERK1/2 in unstimulated or stimulated BV-2 microglia by 50 ng/ml LPS for 15 min in the absence or presence of 5 μM OU126. The bar graphs are the mean ± SE of the intensity of the quantitated phosphorylated forms divided by the non-phophorylated forms of three independent experiments. i The involvement of TRIF and MyD88 pathways in activation of cPLA2α in the signaling leading to CD40 upregualtion. The BV-2 cells were incubated with TRIF or MyD88 peptide inhibitors for 60 min before stimulation with 50 ng/ml LPS for 15 min. A representative immunoblot analysis of cPLA2α activity, out of three independent experiments is presented. The intensity of phosphorylated cPLA2α (p-cPLA2α Ser-505) was quantitated by densitometry as described in A. j Shown is a representative immunofluorescence analysis of CD40 protein expression in the cells treated as in i. DAPI staining shows cell nuclei. Scale bars = 50 μm. The intensity of cPLA2α and of CD40 was quantitated and expressed in the bar graph as arbitrary units. The bar graph is the mean ± SE from three independent experiments. (***p < 0.0001,**p < 0.001, n.s. not significant)
