Structured abstract
Aims
Sensory neuropathy is central to the development of painful neuropathy, and foot ulceration in patients with diabetes. Currently, available QST devices take considerable time to perform and are expensive. NerveCheck is the first inexpensive ($500), portable QST device to perform both vibration and thermal testing and hence evaluate diabetic peripheral neuropathy (DPN). This study was undertaken to establish the reproducibility and diagnostic validity of NerveCheck for detecting neuropathy.
Methods
130 subjects (28 with DPN, 46 without DPN and 56 control subjects) underwent QST assessment with NerveCheck; vibration perception and thermal testing. DPN was defined according to the Toronto criteria.
Results
NerveCheck’s intra correlation coefficient for vibration, cold and warm sensation testing was 0.79 (95% LOA: −4.20 to 6.60), 0.86 (95% LOA: −1.38 to 2.72) and 0.71 (95% LOA: −2.36 to 3.83), respectively. The diagnostic accuracy (AUC) for vibration, cold and warm sensation testing was 86% (SE: 0.038, 95% CI 0.79 to 0.94), 79% (SE: 0.058, 95% CI 0.68 to 0.91) and 72% (SE: 0.058, 95% CI 0.60 to 0.83), respectively.
Conclusions
This study shows that NerveCheck has good reproducibility and comparable diagnostic accuracy to established QST equipment for the diagnosis of DPN.
Keywords: NerveCheck, quantitative sensory testing, neuropathy, diagnostic device and diabetes
1.1 Introduction
Quantitative sensory testing (QST) has been used for decades for diagnosing and quantifying the severity of DPN (Dyck et al. 1978, Shy et al. 2003, Chong and Cros 2004, Yarnitsky and Granot 2006, Backonja et al. 2009) and painful neuropathy (Yarnitsky and Granot 2006, Hansson et al. 2007, Cruccu et al. 2010, Maier et al. 2010, Haanpaa et al. 2011, Krumova et al. 2012, Moloney et al. 2012). Indeed several guidelines endorse the use of QST for the diagnosis of sensory abnormalities in diabetic neuropathy (Kahn 1992, Shy et al. 2003). QST is an automated psychophysical method used to test vibration and thermal sensation which may help to risk stratify patients for the development of painful neuropathy, foot ulceration and amputation (Malik et al. 2011). Easily deployed and inexpensive tests such as the tuning folk, pin-prick, VibraTip and 10g monofilament can detect moderate to severe sensory loss but for early detection of sensory impairment, particularly in clinical trials, QST is required. It provides standardised and quantified stimuli which enable accurate quantification of sensory deficits (Gruener and Dyck 1994) for vibration, a large fibre measure and thermal threshold testing for the detection of small fibre neuropathy (Dyck et al. 1978, Yarnitsky 1997, Shy et al. 2003, Chong and Cros 2004).
Several QST devices are established and are primarily used in clinical research settings. The Neurothesiometer, VSA 3000 (Medoc), Vibrameter (Somedic), Vibration II (Physitemp) and Sensitometer are handheld devices but only perform vibration testing. CASE IV (WR Medical Electronics) measures the function of both vibration and thermal sensation but is large and expensive, provides a complex output in the form of just noticeable differences (JND) from a set of 25 standardised vibratory levels, and requires trained staff. The TSA-II-NeuroSensory Analyser (Medoc) and Sensor (Medoc) perform thermal testing only, are expensive and require a laptop to operate.
NerveCheck was designed to assess vibration (VPT), cold (CPT), warm (WPT) perception threshold and heat pain threshold (HPT). It costs ~ $500 and is portable (size: 9.5 cm × 6.1 cm × 23.6 cm, weighted only 325 g. including battery) as shown in Figure 1. It applies a series of predefined stimuli over a broad range of intensities (i.e. vibration intensity, heat waveform and ramped stimuli (1 °C/s)) to the skin using the method of levels. For each stimulus, the subject reports whether the stimulus was perceived or not or whether it was painful or not. This method is not dependent on the reaction time of the subject. Thresholds for all four modalities are established within 9–13 minutes.
Figure 1.
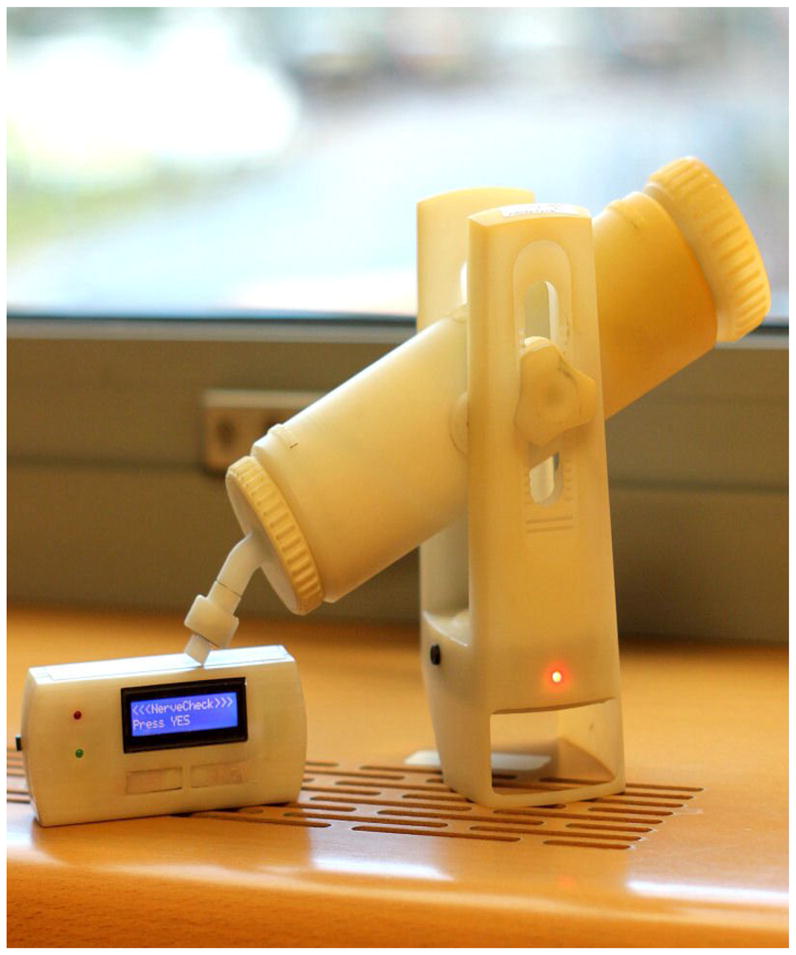
NerveCheck is a portable inexpensive ($500) quantitative sensory device designed to assess vibration (VPT), cold (CPT), warm (WPT) perception threshold and heat pain threshold (HPT). Its size is 9.5 cm × 6.1 cm × 23.6 cm and weights only 325 g. including battery. Its output is categorical in terms of degree of abnormality. The test takes from 3–10 min, depending on whether it is a single test or series of tests.
In the present study we have carefully validated the diagnostic ability of NerveCheck for assessing VPT, CPT and WPT in control subjects and patients with diabetes with a broad range of neuropathy. We have defined the thresholds and examined the reproducibility and diagnostic validity of NerveCheck against other established QST devices.
1.2 Subjects, Materials and Methods
The participants in the study were recruited from the Manchester Diabetes Centre, Manchester Royal Infirmary in Manchester, UK. The study was performed at the NIHR Wellcome Trust Clinical Research Facility between 7 January 2013 and 19 September 2014. Exclusion criteria included subjects with communication disorders, cognitive deficits, severe anxiety, severe depression or history of neuropathy due to a non-diabetic cause. Control subjects suffering from any acute or chronic pain condition were excluded. All subjects were without any pain medication for at least 24 hours before the investigation. This study was approved by the Local Research Ethics committee and all patients gave informed consent to take part in the study. The research adhered to the tenets of the declaration of Helsinki.
1.2.1 Demographic measures
All study participants underwent assessment of their glycated haemoglobin (HbA1c), body mass index (BMI), blood pressure and cholesterol.
1.2.2 Quantitative sensory testing using NerveCheck
Subject were familiarised with the procedure and allowed to acclimatise for 10 minutes in the examination room. NerveCheck (Phi Med Europe S.L. Barcelona, Spain) applies the method of levels where a series of predefined stimuli (in terms of vibration intensity, heat waveform and ramped stimuli (1°C/s)) were applied to the skin and for each stimulus the subject reported whether the stimulus was perceived or not, to establish the vibration (VPT), cold (CPT) and warm (WPT) perception thresholds. There are four kinds of stimuli for vibration and thermal testing. The VPT has void, mild (2.7V), moderate (4.2V) and strong (6.4V) with 9 stimuli in total. The CPT has void, mild (22.4 °C), moderate (17.8 °C) and strong (9.8 °C) with 5 stimuli in total. The WPT has void, mild (37 °C), moderate (39.4 °C) and strong (44.7 °C) with 5 stimuli in total. If the null stimulus answered yes constantly, the result was deemed invalid and was repeated. This method is not dependent on the reaction time of the subject.
The order of administration of stimuli was vibration followed by thermal testing. The stimulator was applied with a constant pressure to the area of skin to be tested. For vibration testing, the vibratory transducer was placed on the dorsal surface of the base of the nail of the great toe. For thermal testing, the thermode (thermoelectric unit with a surface area of 5 cm × 2.5 cm) was placed on the dorsolateral surface of the foot. The thermode provides accurate controlled minute ramps of cooling and heating at the thermode-testing surface using the peltier effect. The administration of cooling and heating stimuli involve gradual changes in temperature along a linear ramp to a preset value and after a specified time return to steady state following an inverse ramp.
The output is categorical in terms of degree of abnormality. The more the subject response correctly to the stimuli the higher the score gets. The normal and abnormal range for VPT is (12–8 & 7–0) and for CPT and WPT is (6–3 & 2–0). The higher the grading score, the more sensitive the participant is to the stimuli. The testing takes 3–13 minutes, depending on whether it is a single test or series of tests. More information about NerveCheck can be found online (http://www.phimedeurope.com/).
1.2.3 Quantitative sensory testing using established devices
Vibration testing was measured using a Neurothesiometer (Horwell, Scientific Laboratory Supplies, Wilford, Nottingham, UK) and was placed at the base of the left great toe. The test was repeated three times and the average value was recorded. Thermal testing including CPT and WPT was undertaken on the dorsum of the left foot using the MEDOC TSA-II-NeuroSensory Analyser (Medoc Ltd. Ramat Yishai 30095, Israel) and method of limits (Fruhstorfer et al. 2003). The test was repeated four times and the average value was recorded.
1.2.4 Neuropathy assessments
All patients underwent an assessment of neuropathy based on a standard protocol including: NDS to classify participants into without (NDS 0–2) and with (NDS 3–10) neuropathy (Abbott et al. 2002, Tesfaye et al. 2010). Electro-diagnostic studies were undertaken using a Dantec “Keypoint” system (Dantec Dynamics Ltd. Bristol, UK) equipped with a DISA temperature regulator to keep limb temperature constantly between 32–35 °C. Peroneal Motor Nerve Conduction Velocity (PMNCV) was assessed in the right lower limb by a consultant neurophysiologist.
1.2.5 Study definition of Diabetic Peripheral Neuropathy
The Toronto Diabetic Neuropathy Expert group recommendation was followed to define DPN: (a) abnormal PMNCV (<42 m/s) (Malik et al. 1990) and (b) abnormal symptoms (NSP) or signs of neuropathy, NDS (>2) (Tesfaye et al. 2010).
1.2.6 Statistical analysis
We estimated that the minimum sample required to detect significant difference between the group with and without vibration sensation loss is 16 subjects and between the group with and without thermal sensation loss is 26. The sample size was calculated by means of an unpaired t-test and with a power of 85%. We examined the distribution of the data by means of relevant histograms and the Shapiro-Wilk test using StatsDirect statistical software, version 2.7.9. All data were expressed as median (5th percentile, 95th percentile). Mann-Whitney U test was performed to analyse differences between the medians. A P value <0.05 was considered statistically significant.
We examined the repeatability of NerveCheck with intraclass agreement using GraphPad Prism, version 6.05. The test–retest intervals were from 1 to 8 weeks references.
Receiver operating characteristic (ROC) curve analysis was used to compare the diagnostic accuracy of NerveCheck against established QST devices using GraphPad Prism, version 6.05. ROC curve analysis established the area under the curve (AUC) to determine the optimal sensitivity and specificity of the NerveCheck test.
1.3 Results
1.3.1 Clinical data
130 subjects (74 with diabetes mellitus (DM) (59 Type 1 DM and 15 Type 2 DM) with median age 55.7 (interquartile range – IQR: 42.9–66.1) and 56 control subjects with median age 43.6 (interquartile range – IQR: 35.7–53.1) were studied. Of the diabetic subjects 28 were diagnosed with and 46 without diabetic peripheral neuropathy (DPN) based on the Toronto criteria. The demographic and clinical characteristics of the participants with and without DPN and controls are presented in Table 1. BMI, HbA1c and cholesterol levels did not differ between the groups with and without DPN, but age (P<0.0001), duration of diabetes (P<0.0001) and systolic blood pressure (P=0.0005) were significantly greater in those with DPN. The group with DPN had a significantly higher Neuropathy disability score (NDS) (P<0.0001) and significantly lower peroneal motor nerve conduction velocity (PMNCV) (P<0.0001). In the NerveCheck tests, the group with DPN had a significantly lower score for vibration perception threshold (VPT) (P<0.0001), cold perception threshold (WPT) (P<0.0001) and warm perception threshold (WPT) (P<0.0001) compared to those without DPN.
Table 1.
Comparison of clinical data of patients with type 1 & 2 diabetes according to the presence or absence of diabetic peripheral neuropathy (DPN) defined by the Toronto criteria and control subjects.
| Variables | Neuropathy | No neuropathy | Controls |
|---|---|---|---|
|
|
|||
| n | 28 | 46 | 56 |
| Gender (male/female) | 25/17 | 22/10 | 26/44 |
| Age | 65.6 (42.5, 83.2) ≡ | 46.48 (23.5, 71.9) | 43.6 (31, 70.3) ≡ |
| Diabetes duration (years) | 46 (4, 60) ≡ | 21 (7, 54) | |
| BMI (Kg/m2) | 26.72 (24, 40.4) | 27.13 (21, 37) | 25 (19.5, 38,8)− |
| Systolic BP (mmHg) | 151 (116, 218)= | 128 (99, 183) | 121 (94, 163) ≡ |
| HbA1c % [mmol/mol] | 8.1 (5.3, 11.2) [65.03 (34, 99)] |
7.64 (6.5, 9.5) [60 (47, 80.1)] ≡ |
5.47 (2.6, 6.2) [36.30 (5.2, 44.3)] ≡ |
| Cholesterol (mmol/l) | 4.5 (3, 6.4) | 4.1 (3, 6.4) ≡ | 4.95 (3.9, 6.4)− |
| NDS | 6 (2, 10) ≡ | 0 (0, 5)− | 0 (0, 3) ≡ |
| PMNCV (m/s) | 36.4 (19, 44.5) ≡ | 44.4 (39.6, 50) ≡ | 48.95 (44.3, 56.5) ≡ |
| VPT (NerveCheck grading) | 0 (0, 12) ≡ | 7 (0, 12)− | 11.34 (3.48, 12) ≡ |
| CPT (NerveCheck grading) | 2.5 (0, 6) ≡ | 6 (3, 6) − | 6 (6, 6) ≡ |
| WPT (NerveCheck grading | 3 (0, 6) ≡ | 6 (2, 6) | 6 (1, 6) ≡ |
Data are medians (5th percentile, 95th percentile), P values are derived from a Mann-Whitney U test: P≤0.05 (−), P≤0.001 (=), P≤0.0001 (≡), The P values for subjects with vs without DPN are in the left column, subjects without DPN vs control subjects in the middle column and subjects with DPN vs control subjects in the right column.
1.3.2 NerveCheck defined threshold values
To define a threshold value of the NerveCheck grading score for VPT, CPT and WPT we have used a mean minus 2 standard deviation (SD) cut-off, based on our control population (n=56) The normal range of the NerveCheck for VPT, CPT and WPT is 4–12 for VPT, 3–6 for CPT and 3–6 for WPT. The total grading score for VPT, CPT and WPT is 0–12, 0–6 and 0–6, respectively.
1.3.3 NerveCXheck reproducibility
Controls and subjects with diabetes (n=16) were tested on two separate occasions to examine the intraclass agreement with test–retest intervals ranging from 1 to 8 weeks. The NerveCheck has good reproducibility as the intraclass agreement for VPT, CPT and WPT is 0.79 (95% limits of agreement: −4.20 to 6.60), 0.86 (95% limits of agreement: −1.38 to 2.72) and 0.71 (95% limits of agreement −2.36 to 3.83), respectively.
1.3.4 NerveCheck diagnostic validity for diabetic peripheral neuropathy
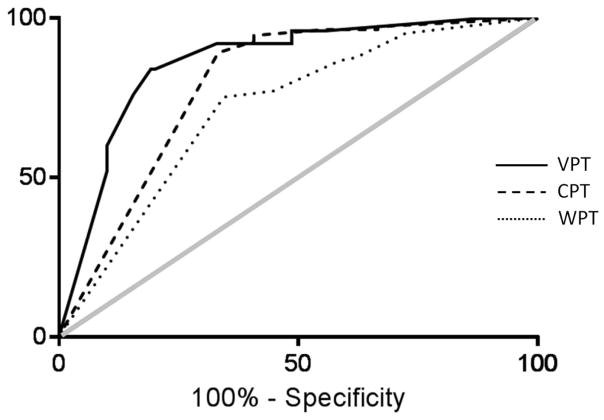
The diagnostic performance of the NerveCheck in detecting sensory loss measured against established QST devices is expressed in AUC using ROC curve analysis (Figure 2). The VPT and thermal testing were compared against the Neurothesiometer and TSA-II-NeuroSensory Analyser (Medoc), respectively. The AUC for VPT is 86% (SE: 0.038, 95% CI 0.79 to 0.94, P<0.0001), for CPT 79% (SE: 0.058, 95% CI 0.68 to 0.91, P<0.0001) and for WPT 72% (SE: 0.058, 95% CI 0.60 to 0.83, P<0.0004).
Figure 2.
ROC curve analysis was used to evaluate the diagnostic performance of Nervecheck in detecting sensory loss against the Neurothesiometer for vibration perception threshold (VPT) (black line) and the TSA-II-NeuroSensory Analyser (Medoc) for cold perception threshold (CPT) (dashed line) and warm perception threshold (WPT) (dotted line) as reference methods for QST. The grey line is the null value of the ROC curve. The AUC for VPT was 86% (SE: 0.038, 95% CI 0.79 to 0.94, P<0.0001), for CPT 79% (SE: 0.058, 95% CI 0.68 to 0.91, P<0.0001) and for WPT 72% (SE: 0.058, 95% CI 0.60 to 0.83, P<0.0004).
The VPT of the NerveCheck displayed high sensitivity 84% (95% CI 63.92% to 95.46%) and high specificity 81% (95% CI 72.07% to 87.66%) with a likelihood ratio of 4.36% for the diagnosis of DPN. The CPT exhibited high sensitivity 89% (95% CI 81.72% to 94.23%) and moderate specificity 67% (95% CI 46.04% to 83.48%) with likelihood ratio 2.67% for the diagnosis of DPN. The WPT exhibited high sensitivity 75% (95% CI 65.86% to 83.14%) and moderate specificity 66% (95% CI 45.67% to 82.06%) with likelihood ratio 2.18% for the diagnosis of DPN.
1.4 Discussion
Sensory dysfunction precedes painful neuropathy, foot ulceration and amputation in patients with diabetes (Tesfaye et al. 2010, Malik et al. 2011). Both sensory loss and threshold values can be measured reliably by quantitative sensory testing (QST) which provides standardised and quantified stimuli and quantifies the level of response (Gruener and Dyck 1994). QST assessment has recently been shown to provide accurate assessment of sensation loss without intra- or inter test differences and has therefore been endorsed as a useful technique for multicenter clinical trials of neuropathy (Dyck et al. 2014). Indeed QST assessment was also recently endorsed for use in the quantification of sensory deficits by the NeuPSIG consensus (Backonja et al. 2013). However, the cost and complex output as well as methodology to establish deficits has hindered more widespread use of QST in the clinic. NerveCheck was designed to measure the vibration (VPT), cold (CPT), warm (WPT) and heat perception threshold (HPT) and provide a simple categorical output which can easily be interpreted in relation to the severity of neuropathy and hence risk stratification. It is the first inexpensive ($500), portable (9.5 cm × 6.1 cm × 23.6 cm) QST device to perform both vibration and thermal testing. We have demonstrated good reproducibility and validity of NerveCheck for assessing sensory loss compared to established devices and give its far lower cost we would suggest much wider use in the clinic. Specifically, NerveCheck has good reproducibility and its ability to detect sensory loss is highly comparable to established QST devices.
When interpreting individual QST results based on the normal range in the NerveCheck grading score, age should be taken into consideration (Dyck 1986). The normal range of the NerveCheck for VPT, CPT and WPT in the current study was based on our control population with a median age of 43.6. The normal range in the NerveCheck grading score applies to the age range 30–72. Indeed there are very few large-scale reference data sets for QST in older adults (Magerl et al. 2010). Both vibration and thermal testing have been reported to be independent of gender (Rolke et al. 2006). In the current study we did not evaluate thermal allodynia or hyperalgesia, as the available normal range for these heat pain thresholds is highly variable (Petersen and Rowbotham 2006, Hansson et al. 2007).
The reproducibility of QST has been a challenge with a significant variability between sessions in the same patient. Indeed in a very early study over 30 years ago, Fagius et al. reported a difference of 150% between assessments using the method of limits (Fagius and Wahren 1981). This problem appears less marked though with the method of levels (Jamal et al. 1985) and NerveCheck uses the method of levels where it applies a series of predefined stimuli over a broad range of intensities. For each stimulus, the subject reports whether the stimulus was perceived or not or whether it was painful or not and is not dependent on the reaction time of the subject. Null stimuli have been included in the algorithms to reduce bias related to a false response. The vibration testing of NerveCheck produces low variability and a factor that may be relevant is that the vibration stimulator is applied with a constant pressure to the area of skin to be tested. Additionally, NerveCheck runs the vibration testing before the thermal testing as assessment of thermal sensation before vibration testing has been found to increase the risk of vibration hyperalgesia (Grone et al. 2012). Of relevance, several studies have shown good reproducibility of VPT (intraclass correlation >0.55) in control subjects and in various patient populations (Chong and Cros 2004, Geber et al. 2011). All said, the thermode of the NerveCheck is a highly engineered device that can provide cooling and heating stimuli along a linear ramp to a preset value at the thermode testing surface using the peltier mechanism as described by Dyck et al. (Gruener and Dyck 1994).
QST is an effective technique for the diagnosis of sensory neuropathy and also provides a composite of quantitative measures, which can be deployed to define the severity of neuropathy. Vibration deficits in the feet suggest large fibre dysfunction (Backonja et al. 2013) and cold and warm deficits indicate small fibre dysfunction (Backonja et al. 2013). Dysfunction of small nerve fibres is thought to be responsible for many painful peripheral neuropathies (Hafner et al. 2015). These small fibre neuropathies cannot be evaluated using standard electrophysiological testing (Dyck et al. 1978, Yarnitsky 1997, Shy et al. 2003, Chong and Cros 2004). Our study shows that NerveCheck has both high sensitivity 84% and high specificity 81% for vibration testing and high sensitivity and moderate specificity for thermal testing. Of relevance, studies have reported variable sensitivity of thermal testing depending on the severity of neuropathy, 27–98% for cold and 22–98% for warm deficits (Chong and Cros 2004), while vibration testing has 58–84% sensitivity and 51–86% specificity (Bril and Perkins 2002, Martin et al. 2010).
NerveCheck provides a cost effective means to identify deficits in vibration and thermal sensation. Both Medoc TSA-II NeuroSensory Analyser and NerveCheck detect sensory deficits. The former test provides the patient’s perception threshold for cold and warm. However, unlike TSA-II-NeuroSensory Analyser, the NerveCheck indicates whether the results are normal or abnormal and stratifies the severity of sensory loss. Additional evaluation may include an assessment of Neuropathic Impairment Score (NIS), autonomic dysfunction via the Neuropad or the assessment of heart rate variability to deep breathing (Ponirakis et al. 2014, Ponirakis et al. 2015) and small fibre structural damage using corneal confocal microscopy (CCM) or skin punch biopsy for intra-epidermal nerve fibre density (IENFD) (Petropoulos et al. 2014, Chen et al. 2015).
In conclusion this is a small but detailed study showing that NerveCheck has good reproducibility and good diagnostic accuracy for assessing sensory loss compared to established QST devices. Clearly larger, prospective studies confirming the diagnostic ability of NerveCheck are required in diabetic neuropathy and in other neuropathies. NerveCheck is the first inexpensive, portable QST device to perform both vibration and thermal testing and therefore provides new opportunities for use in the clinic. It could therefore be deployed as an inexpensive but accurate diagnostic test for diabetic neuropathy in primary care in the developed world and throughout the developing world; which is set to see an explosion in diabetes and hence diabetic neuropathy. It could also be deployed in countries such as India and Brazil for conditions such as leprosy.
Highlights.
Nervecheck is the first inexpensive ($500), portable quantitative sensory testing (QST) device to measure sensory loss.
Nervecheck measures the vibration, cold, warm perception threshold and heat pain threshold, and provide a simple categorical output which can easily be interpreted in relation to the severity of neuropathy and hence risk stratification.
Nervecheck has good reproducibility and comparable diagnostic accuracy to established QST equipment for the diagnosis of diabetic peripheral neuropathy.
Acknowledgments
The NerveCheck device was provided by Dr. Ariel Odriozola, Phi Med Europe S.L. Barcelona, Spain.
We thank the staff at NIHR/Wellcome Trust Clinical Research Facility in Central Manchester University Hospitals NHS Foundation Trust and its Deputy Director, Mr. Paul Brown for providing a high quality service and their state-of-the-art facility to carry out the research. Special thank you to the Nurse Manager, Mr Ciaran Kilkelly and the study Lead Nurse, Mr. Stephen Mawn and Mr. Kamlesh Patel who offered a very professional service to our study subjects and for always being extra helpful.
Funding
This study was funded by Phi Med Europe S.L. Barcelona, Spain, the National Institute of Health (NIH) Grant 5RO1 NS46259-03 NINDS and the Juvenile Diabetes Research Foundation (JDRF) Grant 5-2002-185.
Footnotes
Author contributions
G.P. researched data and wrote the manuscript. A.O, M.N.O, S.O, A.M and M.B.O designed and created the NerveCheck. I.N.P, S.A, H.F, U.A, O.A, A.M, A.K and A.A researched data. R.A.M. and A.O. sponsored and lead the study, reviewed and edited the manuscript. R.A.M. is the guarantor of this work.
Conflicts of Interest
We wish to draw the attention of the Editor to the following facts which may be considered as potential conflicts of interest and to financial contributions to this work. Phi Med Europe S.L. provided the Nervecheck device, funded half of the study and covered some of the travel expenses and conference registration fees for presenting the results of this study. M.N. Odriozola, S. Odriozola, M.B. Odriozola and A. Odriozola are the owners and inventors of the NerveCheck.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, Hann AW, Hussein A, Jackson N, Johnson KE, Ryder CH, Torkington R, Van Ross ERE, Whalley AM, Widdows P, Williamson S, Boulton AJM. The North-West Diabetes Foot Care Study: Incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabetic Medicine. 2002;19(5):377–384. doi: 10.1046/j.1464-5491.2002.00698.x. [DOI] [PubMed] [Google Scholar]
- Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, Edwards RR, Freeman R, Gracely R, Haanpaa MH, Hansson P, Hatem SM, Krumova EK, Jensen TS, Maier C, Mick G, Rice AS, Rolke R, Treede RD, Serra J, Toelle T, Tugnoli V, Walk D, Walalce MS, Ware M, Yarnitsky D, Ziegler D. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain. 2013;154(9):1807–1819. doi: 10.1016/j.pain.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Backonja MM, Walk D, Edwards RR, Sehgal N, Moeller-Bertram T, Wasan A, Irving G, Argoff C, Wallace M. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain. 2009;25(7):641–647. doi: 10.1097/AJP.0b013e3181a68c7e. [DOI] [PubMed] [Google Scholar]
- Bril V, Perkins BA. Comparison of vibration perception thresholds obtained with the Neurothesiometer and the CASE IV and relationship to nerve conduction studies. Diabet Med. 2002;19(8):661–666. doi: 10.1046/j.1464-5491.2002.00759.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Graham J, Dabbah MA, Petropoulos IN, Ponirakis G, Asghar O, Alam U, Marshall A, Fadavi H, Ferdousi M, Azmi S, Tavakoli M, Efron N, Jeziorska M, Malik RA. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care. 2015;38(6):1138–1144. doi: 10.2337/dc14-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong PS, Cros DP. Technology literature review: quantitative sensory testing. Muscle Nerve. 2004;29(5):734–747. doi: 10.1002/mus.20053. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Sommer C, Anand P, Attal N, Baron R, Garcia-Larrea L, Haanpaa M, Jensen TS, Serra J, Treede RD. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17(8):1010–1018. doi: 10.1111/j.1468-1331.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- Dyck P. Detection Thresholds of Cutaneous Sensations in Health and Disease in Man. In: Yaksh T, editor. Spinal Afferent Processing. Springer; US: 1986. pp. 345–362. [Google Scholar]
- Dyck PJ, Argyros B, Russell JW, Gahnstrom LE, Nalepa S, Albers JW, Lodermeier KA, Zafft AJ, Dyck PJ, Klein CJ, Litchy WJ, Davies JL, Carter RE, Melton LJ, 3rd N. T. members of the Cl versus. Multicenter trial of the proficiency of smart quantitative sensation tests. Muscle Nerve. 2014;49(5):645–653. doi: 10.1002/mus.23982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck PJ, I, Zimmerman R, O’Brien PC, Ness A, Caskey PE, Karnes J, Bushek W. Introduction of automated systems to evaluate touch-pressure, vibration, and thermal cutaneous sensation in man. Ann Neurol. 1978;4(6):502–510. doi: 10.1002/ana.410040605. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wahren LK. Variability of sensory threshold determination in clinical use. J Neurol Sci. 1981;51(1):11–27. doi: 10.1016/0022-510x(81)90056-3. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H, Harju EL, Lindblom UF. The significance of A-delta and C fibres for the perception of synthetic heat. Eur J Pain. 2003;7(1):63–71. doi: 10.1016/s1090-3801(02)00056-3. [DOI] [PubMed] [Google Scholar]
- Geber C, Baumgartner U, Fechir M, Vogt T, Birklein F, Treede RD. Comparison of LEP and QST and their contribution to standard sensory diagnostic assessment of spinal lesions: a pilot study. Neurol Sci. 2011;32(3):401–410. doi: 10.1007/s10072-011-0476-9. [DOI] [PubMed] [Google Scholar]
- Grone E, Crispin A, Fleckenstein J, Irnich D, Treede RD, Lang PM. Test order of quantitative sensory testing facilitates mechanical hyperalgesia in healthy volunteers. J Pain. 2012;13(1):73–80. doi: 10.1016/j.jpain.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Gruener G, Dyck PJ. Quantitative sensory testing: methodology, applications, and future directions. J Clin Neurophysiol. 1994;11(6):568–583. [PubMed] [Google Scholar]
- Haanpaa M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, Cruccu G, Hansson P, Haythornthwaite JA, Iannetti GD, Jensen TS, Kauppila T, Nurmikko TJ, Rice AS, Rowbotham M, Serra J, Sommer C, Smith BH, Treede RD. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152(1):14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Hafner J, Lee G, Joester J, Lynch M, Barnes EH, Wrigley PJ, Ng K. Thermal quantitative sensory testing: a study of 101 control subjects. J Clin Neurosci. 2015;22(3):588–591. doi: 10.1016/j.jocn.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Hansson P, Backonja M, Bouhassira D. Usefulness and limitations of quantitative sensory testing: clinical and research application in neuropathic pain states. Pain. 2007;129(3):256–259. doi: 10.1016/j.pain.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Jamal GA, Hansen S, Weir AI, Ballantyne JP. An improved automated method for the measurement of thermal thresholds. 1. Normal subjects. J Neurol Neurosurg Psychiatry. 1985;48(4):354–360. doi: 10.1136/jnnp.48.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R. Proceedings of a consensus development conference on standardized measures in diabetic neuropathy. Quantitative sensory testing. Diabetes Care. 1992;15(8):1092–1094. [PubMed] [Google Scholar]
- Krumova EK, Geber C, Westermann A, Maier C. Neuropathic pain: is quantitative sensory testing helpful? Curr Diab Rep. 2012;12(4):393–402. doi: 10.1007/s11892-012-0282-7. [DOI] [PubMed] [Google Scholar]
- Magerl W, Krumova EK, Baron R, Tolle T, Treede RD, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain. 2010;151(3):598–605. doi: 10.1016/j.pain.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihofner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uceyler N, Valet M, Wasner G, Treede RD. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150(3):439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Malik R, Veves A, Tesfaye S, Smith G, Cameron N, Zochodne D, Lauria G. Small Fiber Neuropathy: Role in the diagnosis of Diabetic Sensorimotor Polyneuropathy. Diabetes Metab Res Rev. 2011 doi: 10.1002/dmrr.1222. [DOI] [PubMed] [Google Scholar]
- Malik RA, Masson EA, Sharma AK, Lye RH, Ah-See AK, Compton AM, Tomlinson DR, Hanley SP, Boulton AJ. Hypoxic neuropathy: relevance to human diabetic neuropathy. Diabetologia. 1990;33(5):311–318. doi: 10.1007/BF00403326. [DOI] [PubMed] [Google Scholar]
- Martin CL, Waberski BH, Pop-Busui R, Cleary PA, Catton S, Albers JW, Feldman EL, Herman WH, Group DER. Vibration perception threshold as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the DCCT/EDIC study. Diabetes Care. 2010;33(12):2635–2641. doi: 10.2337/dc10-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney NA, Hall TM, Doody CM. Reliability of thermal quantitative sensory testing: a systematic review. J Rehabil Res Dev. 2012;49(2):191–207. doi: 10.1682/jrrd.2011.03.0044. [DOI] [PubMed] [Google Scholar]
- Petersen KL, Rowbotham MC. Quantitative sensory testing scaled up for multicenter clinical research networks: a promising start. Pain. 2006;123(3):219–220. doi: 10.1016/j.pain.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Petropoulos IN, Alam U, Fadavi H, Marshall A, Asghar O, Dabbah MA, Chen X, Graham J, Ponirakis G, Boulton AJ, Tavakoli M, Malik RA. Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2014;55(4):2071–2078. doi: 10.1167/iovs.13-13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponirakis G, Fadavi H, Petropoulos IN, Azmi S, Ferdousi M, Dabbah MA, Kheyami A, Alam U, Asghar O, Marshall A, Tavakoli M, Al-Ahmar A, Javed S, Jeziorska M, Malik RA. Automated Quantification of Neuropad Improves Its Diagnostic Ability in Patients with Diabetic Neuropathy. J Diabetes Res. 2015;2015:847854. doi: 10.1155/2015/847854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponirakis G, Petropoulos IN, Fadavi H, Alam U, Asghar O, Marshall A, Tavakoli M, Malik RA. The diagnostic accuracy of Neuropad for assessing large and small fibre diabetic neuropathy. Diabet Med. 2014;31(12):1673–1680. doi: 10.1111/dme.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Shy ME, Frohman EM, So YT, Arezzo JC, Cornblath DR, Giuliani MJ, Kincaid JC, Ochoa JL, Parry GJ, Weimer LH Therapeutics and N. Technology Assessment Subcommittee of the American Academy of. Quantitative sensory testing: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2003;60(6):898–904. doi: 10.1212/01.wnl.0000058546.16985.11. [DOI] [PubMed] [Google Scholar]
- Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnitsky D. Quantitative sensory testing. Muscle Nerve. 1997;20(2):198–204. doi: 10.1002/(sici)1097-4598(199702)20:2<198::aid-mus10>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Granot M. Chapter 27 Quantitative sensory testing. Handb Clin Neurol. 2006;81:397–409. doi: 10.1016/S0072-9752(06)80031-X. [DOI] [PubMed] [Google Scholar]