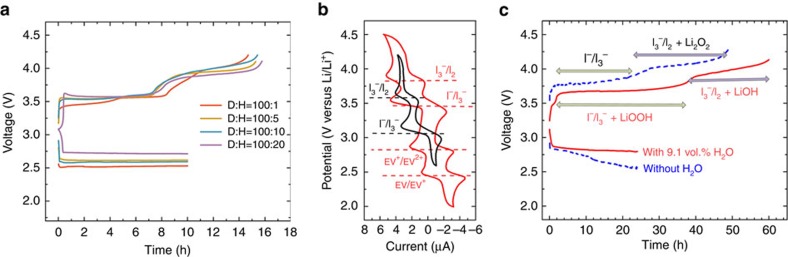
Figure 4. Electrochemical performance of Li-O2 cells with different H2O content in the catholyte.
(a) The charge–discharge curves of water-contaminated Li-O2 batteries. The catholyte consisted of 0.5 ml 50 mM LiI and 0.5 M LiTFSI in DME. Different amount of water was introduced in the catholyte. D:H denotes the volume ratio of DME and H2O. (b) The cyclic voltammograms of 2.5 mM LiI in 0.5 M LiTFSI/DME and 2.5 mM EVI2 in 0.5 M LiTFSI/ DEGDME-DMSO (1:1 v/v). Pt disc and plate were used as the working and counter electrode, respectively. The scan rate was 0.02 V s−1. (c) The charge–discharge curves of redox flow Li-O2 battery using EVI2 as the redox mediator. The catholyte consisted of 4 ml 15 mM EVI2 and 0.5 M LiTFSI in DEGDME-DMSO (1:1 v/v) with or without 9.1 vol.% H2O. The anolyte was 0.5 M LiTFSI in DEGDME. The current was set at 0.1 mA cm−2 for all the above galvanostatic measurements.