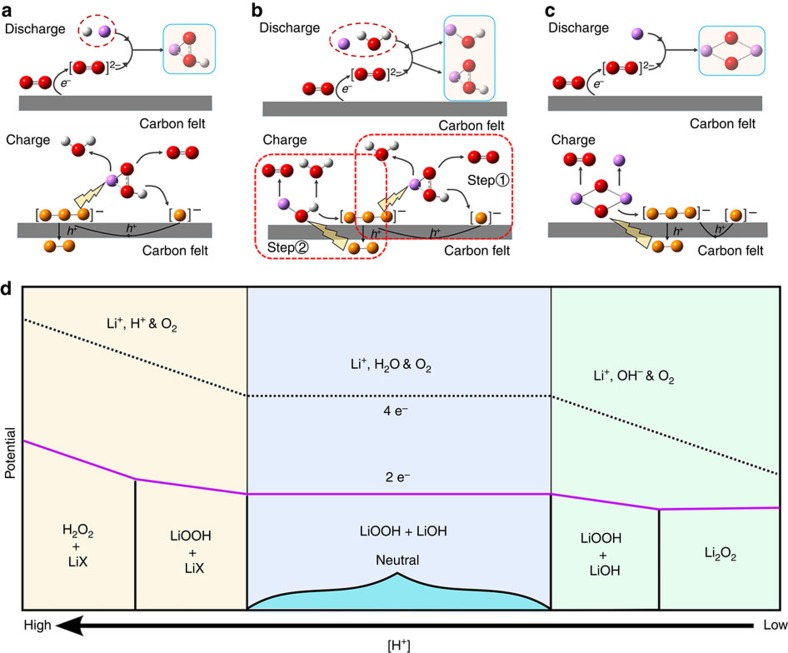
Figure 5. The proposed mechanism of proton-contaminated Li-O2 batteries and Pourbaix diagram with different proton concentration.
The proposed mechanisms of the charging and discharging processes in ‘proton-contaminated' aprotic Li-O2 battery at (a) acidic, (b) neutral and (c) basic conditions. Iodide is included to mediate the OER reaction. Elements in the ball-and-stick model: red-oxygen; purple-lithium; write-hydrogen; yellow-iodine. (d) A sketch of the Pourbaix diagram showing the predominant battery chemistries of Li-O2 cell at different [H+]. The 4-electron process shown in dotted line is just for reference and the potential relative to that of the two-electron process has no physical significance. HX is the acid introduced in the electrolyte.