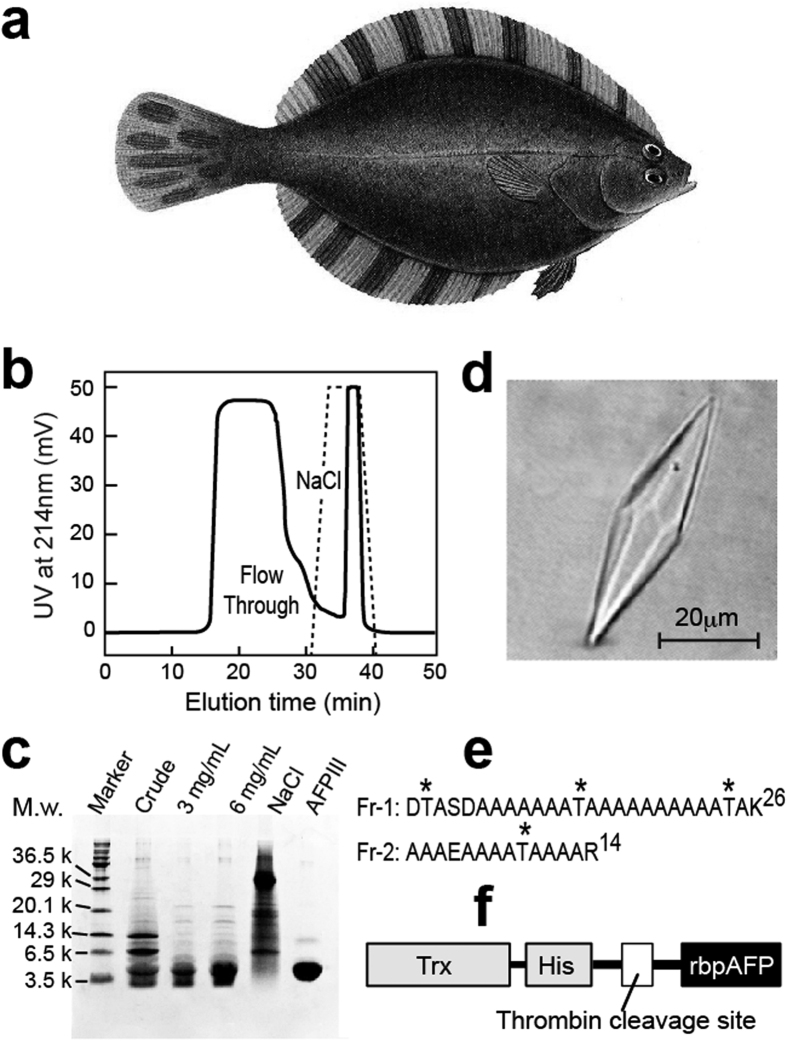
Figure 1.
Purification of AFP from barfin plaice and bpAFP sequence: (a) Illustration of Liposetta pinnifasciata (barfin plaice) copied with permission from ‘An Illustrated Book of Fish’ (p.922, #3688), Hokuryukan Co. Ltd, Tokyo, Japan. (b) DEAE anion-exchange chromatography of partially purified bpAFP, where the AFP appeared in the large flow-through peak and contaminants were separately eluted with NaCl (dashed line). (c) Electrophoretogram of fractions from the anion-exchange chromatography step separated by 15% Tris-Glycine SDS-PAGE. Samples analyzed were the starting material for the chromatography (crude); two loadings of the flow through peak; contaminants eluted by NaCl; and type III AFP used as a molecular weight standard for 7 kDa. (d) Hexagonal trapezohedral ice crystal formed in 0.25 mg·mL−1 of the bpAFP solution. (e) Sequence of the two tryptic fragments (Fr-1 and -2) obtained from the HPLC-purified bpAFP, where asterisks mark threonines with the 11-residue periodicity. (f) Schematic diagram of the fusion protein construct composed of thioredoxin (Trx), His-tag, and a thrombin cleavage site used for the expression of rbpAFP.