Abstract
Nilaparvata lugens (Stål) (Hemiptera: Delphacidae) is a major rice pest that harbors an endosymbiont ascomycete fungus, Entomomyces delphacidicola str. NLU (also known as yeast-like symbiont, YLS). Driving by demand of novel population management tactics (e.g. RNAi), the importance of YLS has been studied and revealed, which greatly boosts the interest of molecular level studies related to YLS. The current study focuses on reference genes for RT-qPCR studies related to YLS. Eight previously unreported YLS genes were cloned, and their expressions were evaluated for N. lugens samples of different developmental stages and sexes, and under different nutritional conditions and temperatures. Expression stabilities were analyzed by BestKeeper, geNorm, NormFinder, ΔCt method and RefFinder. Furthermore, the selected reference genes for RT-qPCR of YLS genes were validated using targeted YLS genes that respond to different nutritional conditions (amino acid deprivation) and RNAi. The results suggest that ylsRPS15p/ylsACT are the most suitable reference genes for temporal gene expression profiling, while ylsTUB/ylsACT and ylsRPS15e/ylsGADPH are the most suitable reference gene choices for evaluating nutrition and temperature effects. Validation studies demonstrated the advantage of using endogenous YLS reference genes for YLS studies.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR) is the most popular and indispensable tool in molecular biology1,2. RT-qPCR assay allows fast, accurate and sensitive measurement of target mRNA during the exponential amplification phase by detecting fluorescent markers directly related to the amount of amplified target. The technique combines amplification, detection and quantification in a single step. RT-qPCR assay measures cycle threshold (Ct, also referred as quantification cycle or crossing point that is abbreviated as Cq or Cp), defined as the PCR cycles at which the fluorescent signal of the reporter dye crosses an arbitrarily set threshold. One direct and precise quantification method is using standard curves in which samples of known quantity are serially diluted and then amplified. The generated standard curve can then be used to quantify samples of unknown quantity. However, the most popular tool is comparative RT-qPCR, where the quantification is achieved through Ct difference (ΔCt) between the gene of interest and a housekeeping gene(s) (also named as reference gene or normalizing gene) in the same sample without knowing the absolute level of expression3.
The ΔCt method requires the chosen reference gene(s) to have an almost constant level of expression across all conditions/samples, because Ct values can be influenced by variations in RNA extraction (RNA quality and quantity), reverse transcription, cDNA concentration, and PCR reaction (including components, parameters and reaction efficiency)4. To obtain reliable and valid gene expression profiles, stable internal controls are critical for normalizing expression1. Reference genes are mostly selected from housekeeping genes (HKGs) because they are essential to the basic cellular survival and normally are expressed constitutively in all cells regardless of conditions (tolerant to regulations)1,2,5,6,7,8. A variety of HKGs have been evaluated and utilized as reference genes in different organisms5,9,10. For examples, γ-globin, NADH oxidase (NOX), aldolase (ALD), hydroxymethylbilane synthase, hypoxanthine phosphoribosyl transferase 1, β-actin (ACTB), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 18S ribosomal RNA (18S rRNA) have been validated and used extensively as reference genes for expression studies of many different organisms10,11,12,13,14, and other genes (e.g. ubiquitin 10 and tubulin 6, that were abbreviated as UBQ10 and TUB6, respectively) have been ruled unsuitable as references15. According to the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines16, HKGs are now selected based on their specificity in interactions between a species or cell type/s subjected to different treatments or conditions. This recommendation requires more detailed expression characterization of potential HKGs in various samples and conditions. More recent, the increasing popularity of the high-throughput next generation sequencing technologies (HT-NGS) has led to a significant increase in transcriptomic and genomic resources of various organisms17,18,19,20. RT-qPCR is one powerful and widely used tool to characterize and assess the quality of these genetic data1,2, making studies of potential reference gene characteristics more urgent.
The brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae), is a major pest of rice in Asia21,22,23,24. Novel population management tactics are in high demand. N. lugens harbors an endosymbiont ascomycete fungus, Entomomyces delphacidicola str. NLU (also known as yeast-like symbiont, YLS) that has been revealed to play an important role in amino acid biosynthesis for N. lugens25. In recent years, new technologies such as NGS and RNA interference (RNAi) offer the possibility of understanding the molecular principles of N. lugens and YLS interaction, and provide potential opportunities for novel pest management24,26,27 as high mortality can be resulted from double-stranded RNA (dsRNA)-mediated knockdown of N. lugens originated genes28,29,30,31,32,33. Similarly, dsRNA knockdowns of YLS originated genes offer another valid population management option that can provide extra safety for non-target organisms with no YLS34. Our laboratory has identified YLS originated genes involved in essential amino acid biosynthesis pathways for N. lugens that could potentially serve as candidates for RNAi34,35. Although N. lugens originated housekeeping genes including ribosomal protein S11e (NlRPS11), NlRPS15, α2-tubulin (NlTUB) and β-actin (NlACT) have been used as reference genes for YLS studies34, using endogenous YLS genes would be more convincible for accurate expression profiling.
In this study, we identified and cloned eight previously unreported YLS genes as reference gene candidates. The expression stabilities of these genes were evaluated for N. lugens samples of difference developmental stages and sexes, and under different temperatures and nutritional conditions. Furthermore, the suitability of these genes as reference controls for RT-qPCR assays of YLS genes that respond to different nutritional conditions (amino acid deprivation) and RNAi were evaluated.
Results
Expression of YLS housekeeping gene candidates
Using the pooled insect samples, the expression of the eight selected HKGs of YLS was investigated by RT-qPCR. All genes were expressed and visualized as a single band of the expected sizes on 2% agarose gels. All amplicons were sequenced and displayed 99–100% identity with the corresponding gene sequences. The PCR efficiency (calculated from the standard curve) and correlation coefficient (R2) are shown in Table 1. The primer efficiency ranges between 93% and 105% with R2 of 0.995–1.000. These data indicate that amplification efficiencies of the primers met the standard requirement of conventional RT-qPCR (efficiency between 90% and 110% with R2 of 0.985–1.000).
Table 1. Primers used for RT-qPCR analysis and dsRNA synthesis.
| Gene | Primer sequence (5′ to 3′) | Amplicon size (bp) | PCR efficiency (%) | R2 |
|---|---|---|---|---|
| RT-qPCR | ||||
| ylsAct | CATCCACGTAACCACCTACAA | 224 | 105.4 | 0.999 |
| AGGCCAAGATAGATCCACCA | ||||
| ylsEF1α | ACTACCCAGTGGTCCGAGTC | 209 | 104.1 | 1.000 |
| AAGGTCTTGCCAGTGGTCTT | ||||
| ylsGADPH | GCAAAGTCATTCCCGATCTT | 196 | 103.6 | 0.996 |
| GAGACGATTTCTTCATCGCA | ||||
| ylsRPS11 | ACGGAATCCCATTCAAAGAG | 167 | 102.5 | 0.999 |
| AGCTTGAGCAGCTACATTGG | ||||
| ylsRPS15e | AAGCCAAGCCTAACGAGAAG | 234 | 99 | 0.997 |
| GTGGAATGAAACGAGACGAA | ||||
| ylsRPS15p | AGCTCTGAGGTCCAGATTGC | 193 | 103.5 | 1.000 |
| TCAAGTGGGTCCATCTTTCA | ||||
| ylsTub | TTGATTGCTCAGGTCGTCTC | 196 | 115.7 | 0.995 |
| GGTCATCTCTTGGACGGAGT | ||||
| ylsUBQ | CTTTGACGGGAAAGACGATT | 156 | 92.8 | 0.998 |
| AATCGCTCAGAGTACGTCCA | ||||
| NlylsSDH | AATGCCTGCTCGTTGATAAC | 185 | N.A. | N.A. |
| ATGTCTTGCCCTAAGGTGCT | ||||
| ylsArg4 | CGAGTTCGAGCAGATTGAAA | 70 | N.A. | N.A. |
| GATGGCAAATATGCCTGTTG | ||||
| EdePRTase | CGAATCCAGACATGATCGAC | 165 | N.A. | N.A. |
| AGCCGTCATCCAGTGATGTA | ||||
| dsRNA synthesis | ||||
| dsEdePRTase | T7-AAGAATCTACCGATCGCCCT | 532 | N.A. | N.A. |
| T7-GGGATCTAATCAAGACGGCA | ||||
| dsylsArg4-1 | T7-GACATACGCGGCAGCATT | 430 | N.A. | N.A. |
| T7-CGAGGCAGTAGGAAAGAAGC | ||||
| dsylsArg4-2 | T7-TCACCGAGACCCTACAATG | 307 | N.A. | N.A. |
| T7-TCCGACACCGTCTTGACA | ||||
| dsGFP | T7-CCTGAAGTTCATCTGCACCAC | 355 | N.A. | N.A. |
| T7-TGATGCCGTTCTTCTGCTTGT | ||||
Note: T7 RNA polymerase promoter sequence (5′-TAATACGACTCACTATAGGG-3′). N.A., not applicable.
The raw Ct values of all tested samples reveal that these genes were expressed at different levels (average Ct between 19.72 and 30.12) with different levels of variation (standard error, SE between 0.2350 and 0.5021). As shown in Fig. 1, ylsEF1α expressed at the lowest level (the highest Ct value), followed by ylsRPS15p, ylsACT, ylsGAPDH, ylsTUB, ylsRPS15e, ylsPRS11 and ylsUBQ. Among them, YlsTUB, ylsUBQ and ylsEF1α had higher expression variation, whereas the other five genes had lower expression variation (ylsACT < ylsRPS15e < ylsRPS15p < ylsGAPDH < ylsPRS11). The detection of gene specific expression variation of different samples signifies the importance of customizing reference gene selection according to sample types and experimental conditions.
Figure 1. Expression profiles of candidate reference genes in N. lugens.
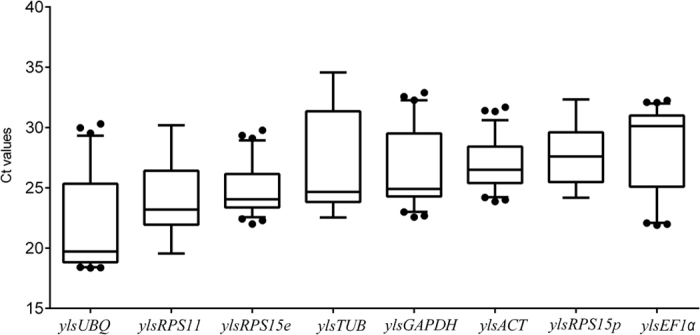
The expression level of candidate references in all tested samples are documented in Ct-value, and showed as boxplot. The dot indicates the maximum or minimum value of Ct, while whiskers indicate the standard error (SE) of the mean.
Stability of reference gene candidates
To identify the most stable reference gene of the different sample types, the expression stabilities were evaluated by the ΔCt method, NormFinder, geNorm and BestKeeper, respectively. The overall stability ranking was obtained by RefFinder.
Development and sex
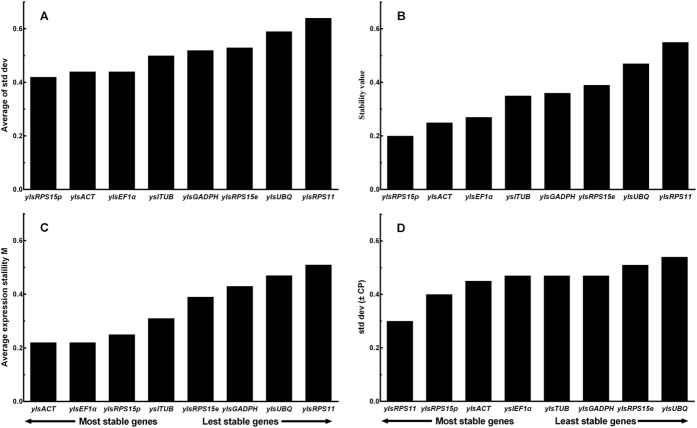
The ΔCt method indicated that ylsRPS15p, ylsACT and ylsEF1α were the most stable reference genes across samples of different developmental stages and sexes (Fig. 2A). The lower expression stability values showed by NormFinder also indicated that ylsRPS15p, ylsACT and ylsEF1α were the most stably expressed genes (Fig. 2B). The estimation of geNorm shows that ylsACT, ylsEF1α and ylsRPS15p were the most stable genes, and ylsPRS11 and ylsUBQ were the most unstable genes (Fig. 2C). Analysis using BestKeeper indicated that ylsACT, ylsEF1α, ylsRPS15p and ylsTUB were the most stable reference genes (Fig. 2D). RefFinder ranks the genes from most to least stable as the following: ylsRPS15p > ylsACT > ylsEF1α > ylsTUB > ylsPRS11 > ylsGAPDH > ylsRPS15e > ylsUBQ (Table 2). Furthermore, geNorm analysis revealed that all pairwise variations (Vn/Vn + 1) were below 0.15 (the recommended threshold of cut-off), indicating that the inclusion of additional reference is unnecessary (Fig. 3). Thus, the best combination of reference genes for developmental stage and sex samples of N. lugens are ylsRPS15p and ylsACT.
Figure 2. Stability of the reference gene expression in different developmental stages of N. lugens.
Candidate genes (ylsActin, ylsEF1α, ylsGADPH, ylsRPS11, ylsRPS15e, ylsRPS15p, ylsTub, ylsUBQ) were evaluated using the comparative ΔCt method (A), NormFinder (B), geNorm (C) and BestKeeper (D).
Table 2. Ranking of the candidate reference genes as determined by RefFinder.
| Conditions | Rank | Gene | Geomean of ranking values |
|---|---|---|---|
| Development stage and sex | 1 | ylsRPS15p | 1.57 |
| 2 | ylsACT | 1.86 | |
| 3 | ylsEF1α | 2.45 | |
| 4 | ylsTUB | 4.23 | |
| 5 | ylsRPS11 | 4.76 | |
| 6 | ylsGADPH | 5.48 | |
| 7 | ylsRPS15e | 5.96 | |
| 8 | ylsUBQ | 7.24 | |
| Artificial diet | 1 | ylsACT | 1.19 |
| 2 | ylsTUB | 1.41 | |
| 3 | ylsEF1α | 3.41 | |
| 4 | ylsRPS15e | 4.00 | |
| 5 | ylsGADPH | 5.44 | |
| 6 | ylsRPS11 | 5.45 | |
| 7 | ylsRPS15p | 6.24 | |
| 8 | ylsUBQ | 8.00 | |
| Temperature | 1 | ylsRPS15e | 1.41 |
| 2 | ylsGADPH | 2.51 | |
| 3 | ylsACT | 2.59 | |
| 4 | ylsTUB | 3.64 | |
| 5 | ylsEF1α | 4.12 | |
| 6 | ylsUBQ | 5.42 | |
| 7 | ylsRPS15p | 5.66 | |
| 8 | ylsRPS11 | 8.00 |
Figure 3. Optimal number of reference genes for normalization in N. lugens.
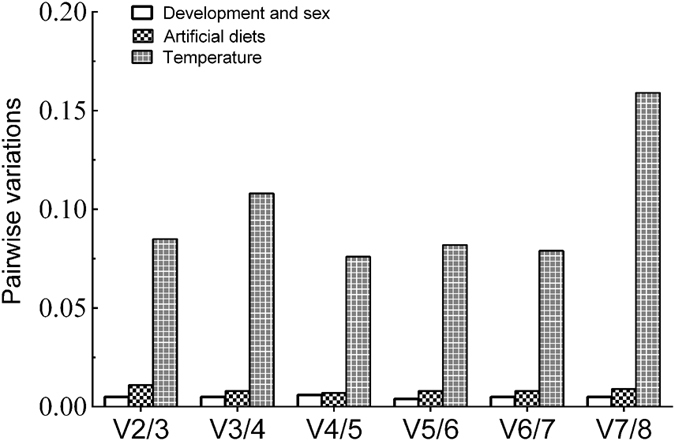
The pairwise variation (Vn/Vn+1) was analyzed by the geNorm software to determine the optimal number of reference genes for RT-qPCR. The average values of pairwise variation (V) dictate whether inclusion of an extra reference gene would add to the stability of the normalization factor.
Different nutritional conditions
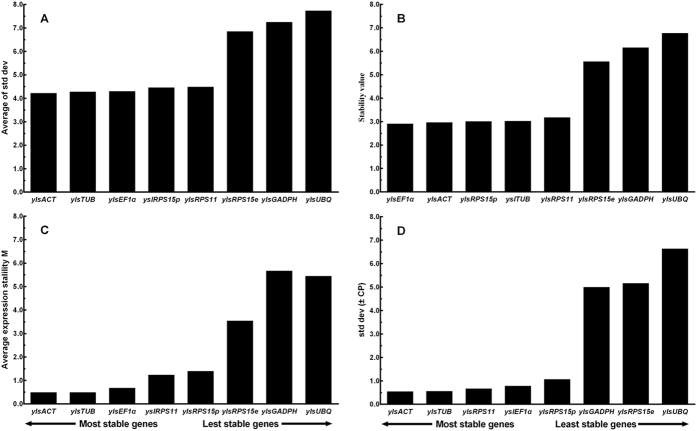
Analyses of ΔCt method, NormFinder and BestKeeper divided the genes into two groups: more stably expressed ylsACT, ylsTUB, ylsEF1α, ylsRPS15p and ylsPRS11; and less stably expressed ylsUBQ, ylsRPS15e and ylsGAPDH (Fig. 4A,B,D). The geNorm analysis divided the genes into three groups: the most stably expressed ylsACT, ylsTUB and ylsEF1α (M < 1); the least stable expressed ylsUBQ, ylsRPS15e and ylsGAPDH (M > 3); and a middle group of ylsPRS11 and ylsRPS15p (1 < M < 3) (Fig. 4C). RefFinder ranking from most to least stable is ylsACT > ylsTUB > ylsEF1α > ylsRPS15e > ylsGAPDH > ylsPRS11 > ylsRPS15p > ylsUBQ (Table 2). The geNorm analysis also revealed that all pairwise variation (Vn/Vn + 1) values were below 0.15 (Fig. 3). Thus, the combination of reference genes recommended for this sample type is ylsTUB and ylsACT.
Figure 4. Stability of the reference gene expression in N. lugens reared on artificial diets that missed one of the 20 amino acids.
Candidate genes (ylsActin, ylsEF1α, ylsGADPH, ylsRPS11, ylsRPS15e, ylsRPS15p, ylsTub, ylsUBQ) were evaluated using the comparative ΔCt method (A), NormFinder (B), geNorm (C) and BestKeeper (D).
Temperature conditions
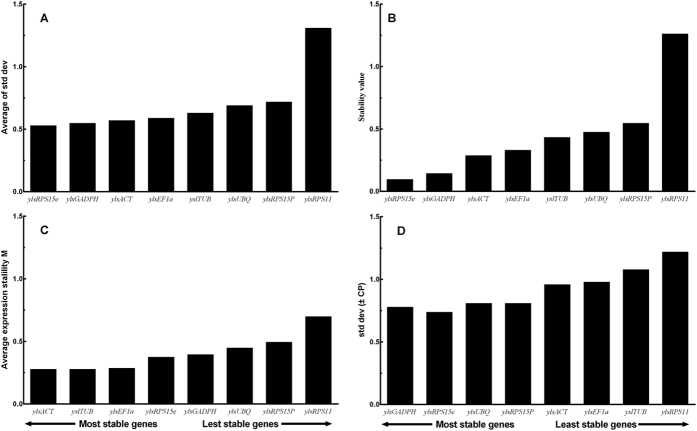
With the samples exposed to different temperature conditions, the comparative ΔCt method, NormFinder and BestKeeper analyses indicated that ylsRPS15e and ylsGAPDH were the most stable genes, while ylsACT and ylsTUB were the most stable genes found by geNorm (Fig. 5). According to RefFinder, the overall order from most to least stable is ylsRPS15e > ylsGADPH > ylsACT > ylsTUB > ylsEF1α > ylsUBQ > ylsRPS15p > ylsRPS11 (Table 2). The geNorm analysis revealed that almost of all pairwise (Vn/Vn + 1) variations were below 0.15 (Fig. 3). Thus, the combination of reference genes recommended for this subset is ylsRPS15e and ylsGADPH.
Figure 5. Stability of the reference gene expression in N. lugens reared on rice under different temperatures.
Candidate genes (ylsActin, ylsEF1α, ylsGADPH, ylsRPS11, ylsRPS15e, ylsRPS15p, ylsTub, ylsUBQ) were evaluated using the comparative ΔCt method (A), NormFinder (B), geNorm (C) and BestKeeper (D).
Validation of reference gene selection
Recently, the temporal expression patterns of saccharopine dehydrogenase (NlylsSDH) and ATP-phosphoribosyltransferase (EdePRTase) were determined by using two N. lugens reference genes NlRPS11 and NlRPS15 as internal controls34,36. In the current study, the relative expression of NlylsSDH, EdePRTase and argininosuccinate lyase (ylsArg4) in N. lugens samples of different developmental stages and sexes was determined using the YLS reference gene pair ylsRPS15p and ylsACT, an identified unstable gene ylsUBQ and the N. lugens reference gene pair NlRPS11 and NlRPS15. Two-way ANOVA was performed to evaluate the effect of the reference genes and the developmental stages. As expected, the expression profiles of the two target genes (NlylsSDH and EdePRTase) showed significant difference among developmental stages, but the expression profile of ylsArg4 was not significant different at P = 0.05 (NlylsSDH: F7, 24 = 4.938, P = 0.0054; EdePRTase: F7, 24 = 6.174, P = 0.0019; ylsArg4: F7, 24 = 2.212, P = 0.0977). Similar to development profiles, reference gene effect was significant for NlylsSDH and EdePRTase but not for ylsArg4 (NlylsSDH: F2, 24 = 4.449, P = 0.0319; EdePRTase: F2, 24 = 4.471, P = 0.0315; ylsArg4: F2, 24 = 1.829, P = 0.1969). Significant interaction effect was found for all target genes (NlylsSDH: F14, 24 = 37.064, P < 0.001; EdePRTase: F14, 24 = 26.201, P < 0.001; ylsArg4: F14, 24 = 27.376, P < 0.001) suggesting that the impact of reference genes varied among samples of different growth stages. Figure 6 shows the effect of different reference genes on the target gene expression levels for each developmental stage.
Figure 6. Validation of reference gene selection in samples of developmental stages and sex.
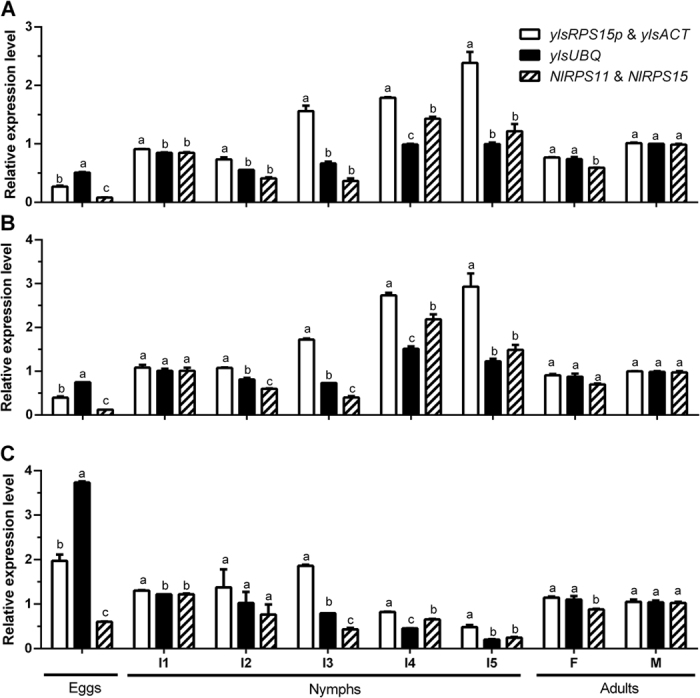
The expression of target genes ylsSDH (A), EdPRTase (B) and ylsArg4 (C) was determined in eggs, nymphs (the first to fifth instar, I1 to I5), female and male adults (F and M), respectively. The data are denoted as mean ± SE. Different letters above the bars represent significant differences among samples of developmental stages and sexes using one of the three reference gene types (P = 0.05) (Turkey’s test).
Furthermore, the mRNA responses of EdePRTase or ylsArg4 to histidine- or methionine-free artificial diets were determined as EdePRTase is involved in the biosynthesis of histidine and ylsArg4 is involved in the biosynthesis of methionine. The results showed that differences were present among the tests of the different reference genes (Fig. 7). For EdePRTase, both reference gene pairs of NlRPS11/NlRPS15 and ylsTUB/ylsACT detected the expected difference of expression between normal and histidine deprived diets (Fig. 7A), while the unsuitable reference gene ylsUBQ failed. Using NlRPS11/NlRPS15 as reference genes, the expression levels of EdePRTase that responded to the histidine-free artificial diet was consistent with published results36. For ylsArg4, only ylsTUB/ylsACT reference gene pair test was able to detect the expected difference between normal and methionine deprived diets (Fig. 7B).
Figure 7. Validation of reference gene selection in samples representing different nutrition diets.
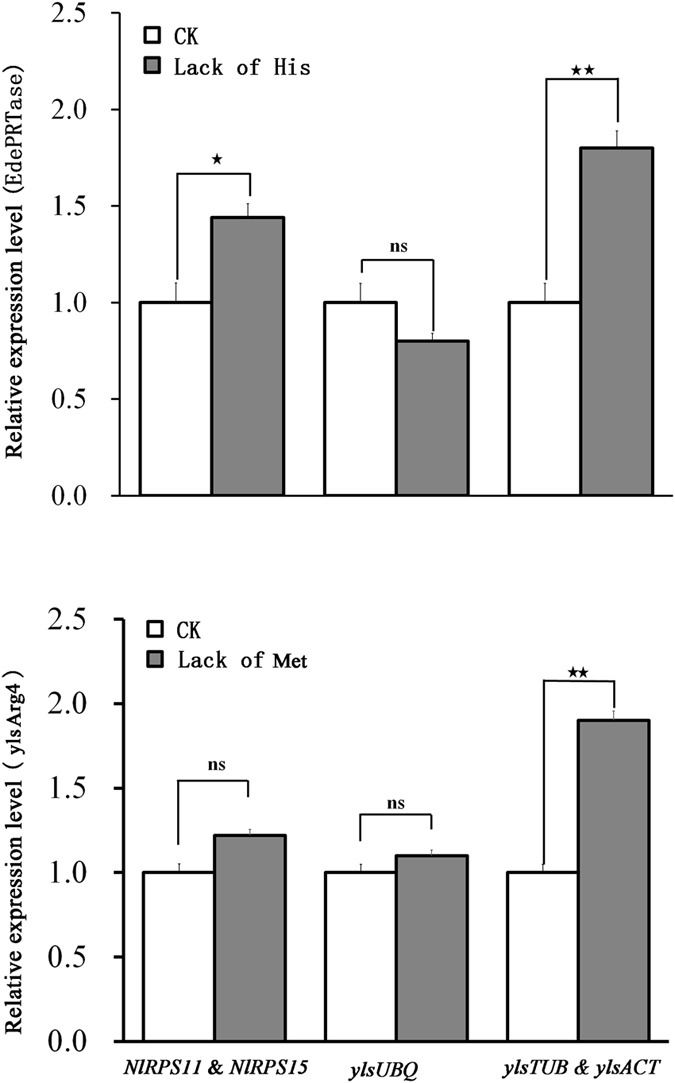
The expression of target genes EdPRTase (A) and ylsArg4 (C) was determined in artificial diet D-97 (CK) and histidine- or methionine-free diet, respectively. The data are denoted as mean ± SE. The pentagram above the vertical bars represent significant differences (P = 0.05) (Student’s t test). Ns, not significant.
All three RNAi samples tested with different reference genes were able to detect EdePRTase knockdowns with similar trends (Fig. 8A). However, the knockdown of ylsArg4-1 was detected by the tests with reference genes of NlRPS11/NlRPS15 and ylsTUB/ylsACT. The knockdown of ylsArg4-2 was only detected by the test with reference genes of ylsTUB/ylsACT. The deemed unsuitable reference gene ylsUBQ failed to detect any knockdowns (Fig. 8B).
Figure 8. Validation of reference gene selection in samples of dsRNA injection.
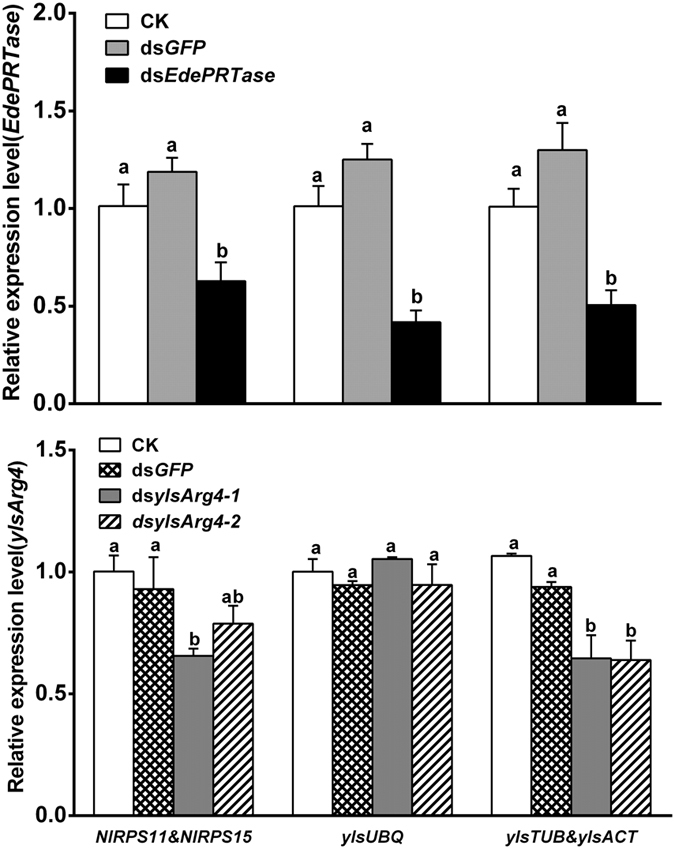
The expression of target genes EdPRTase (A) and ylsArg4 (B) was determined in dsEdePRTase or dsylsArg4 (dsylsArg4-1 and dsylsArg4-2) injected samples, respectively. The data are denoted as mean ± SE. Different letters above the vertical bars represent significant differences (P = 0.05) (Turkey’s test). Ns, not significant.
Discussion
Reference gene(s) selection is a crucial component of RT-qPCR that directly impacts the quality of gene expression profiling. A good reference gene should be expressed consistently regardless of tissue type and experimental condition. Reference genes also need tolerance to differences in starting material amount and quality, and to differences in RNA extraction and cDNA synthesis as they are exposed to the same preparation procedure of the target gene(s)37,38. On the other hand, a gene in living cells cannot be totally immune to changes responding to physiological and nutritional status due to experimental manipulations. It is important to identify the least regulated gene(s) of individual experiment as reference genes for accurate RNA transcription analysis. The current study focused on YLS of N. lugens as YLS is crucial for the survival of the hosts21. Recent studies have identified multiple YLS genes that play a role in essential amino acid biosynthesis34,36,39, which brings more attention to YLS. Eight YLS genes in N. lugens were investigated for their expression stabilities in an effort of identifying suitable reference genes for RT-qPCR studies of holosymbiont (including both N. lugens and YLS, N. lugens-symbiont YLSs). To our knowledge this is the first report of such kind.
Among the eight evaluated YLS genes, ylsRPS15e that encodes one member of ribosomal proteins had the third highest expression level with the penultimate lowest expression variation, comparing to the other genes, suggesting that it would be a good reference gene. A literature search indicates that this is the first report that RPS15e is suggested as a reference gene in fungi. In insects, RPS11 and RPS15 from N. lugens and ribosomal protein L10 and ribosomal protein L17A from Spodoptera exigua were proven to be acceptable reference genes, with high expression in all tissue samples40,41. GAPDH is one of the most commonly used reference genes and is often referred as “classical”42. The use of GAPDH in many studies produced good results, but in some other studies GAPDH was not recommended due to expression variability caused by experimental factors43. Additionally, GAPDH’s roles outside of the glycolytic pathway that may result in variable expression levels of different tissues44,45. These results indicate that genes involved in the fundamental processes of cells as candidate reference genes might also be significantly influenced by other processes during experimentation. In the current study, ylsEF1α and ylsGAPDH showed relatively lower expression levels and higher expression variations, and should be avoided for relative expression analysis in some specific conditions.
For molecular level studies of the interaction between YLS and N. lugens, gene expression of different developmental stages are often characterized as the information is important for gene function analysis and the timing of RNAi. Additionally, nutrition and temperature are important experimental factors because of YLS’s role in amino acid biosynthesis and that YLS as fungus are sensitive to temperature manipulation. For these reasons, we evaluated expression stability of the eight YLS genes across developmental stages and identified the best reference gene pair ylsRPS15p/ylsACT. Similarly, the best reference gene pairs for nutritional and temperature manipulations were identified as ylsTUB/ylsACT, and ylsRPS15e/ylsGADPH, respectively. These results should greatly benefit the RT-qPCR studies of N. lugens or other organisms that host YLS.
Currently the most YLS studies used genes of its host N. lugens, e.g. RPS11 and RPS15, as reference genes regardless of experimental conditions. Our study shows that endogenous reference genes should be selected depending on experimental settings and objectives. To verify our reference gene selections, we conducted comparative studies of the three recommended reference gene systems using the selected target genes. For NlylsSDH (encodes saccharopine dehydrogenase that catalyzes the penultimate reaction in the biosynthesis of the amino acid lysine), ylsRPS15p/ylsACT reference genes had the advantage of detecting higher expression levels in fourth- and fifth-instar nymphs, compared to the reference genes of ylsUBQ and NlRPS11/NlRPS15. Previous study using NlRPS15 and NlTUB as internal references had suggested that NlylsSDH had higher expression levels in first to fourth instar nymphs than that in fifth instar nymph and adults34. However, when RNAi experiment was carried out for the first instar nymphs, no significant adverse effect on body weights and nymphal development durations were observed, suggesting that the gene may not be highly expressed in the 1st instar nymphs. Performing RNAi during the 4th or 5th instar may yield better results. Target gene EdePRTase (also originated from YLS) is ubiquitously but unevenly expressed among the different life stages using the reference genes of N. lugens, NlRPS11 and NlRPS15 36. In the current study, EdePRTase had the highest expression level in the fourth and fifth instar nymphs using all three reference gene systems, which is partially consistent with the published expression patterns36. The EdePRTase RNAi experiments were successful, with significant down-regulated expression of EdePRTase and histidine level patterns.
More importantly, the current study demonstrated the importance of reference gene selections for different experimental objectives. EdePRTase is a gene that contributes to the biosynthesis of histidine. Both YLS and N. lugens reference genes were able to detect the expected expression difference due to the methionine deprived diet. However, for ylsArg4, an YLS gene involved in the biosynthesis of methionine, only ylsTUB/ylsACT reference gene pair was able to detect the expected difference between the normal and methionine deprived diets. Similarly, RNAi experiments showed that gene knockdown of EdePRTase was detected by all the reference genes used with similar trends, while the knockdown of ylsArg4-1 was detected by reference genes of NlRPS11/NlRPS15 and ylsTUB/ylsACT, and the knockdown of ylsArg4-2 was only detected by the use of reference genes of ylsTUB/ylsACT. Thus, YLS genes seem more tolerant to experimental manipulations. These results clearly demonstrate the advantage of using endogenous YLS genes over exogenous host genes NlRPS11 and NlRPS15.
Conclusion
Tailored reference gene selection is deemed necessary for gene transcription studies by RT-qPCR. For organisms hosting microbial endosymbionts, using endogenous genes of the symbionts as reference genes may possess advantages over host genes. The current study concludes that ylsRPS15p and ylsACT are the most suitable reference genes for temporal gene expression profiling, while ylsTUB and ylsACT, and ylsRPS15e and ylsGADPH are the most suitable reference gene choices for evaluating nutrition and temperature effects. Such specific reference gene recommendations should benefit future gene expression studies of N. lugens and its YLS. To our knowledge, this is the first study that investigates different candidate reference genes for gene expression analyses and seems to be useful in guiding researchers in performing gene expression analyses in this species.
Materials and Methods
Ethics statement
All animal work has been conducted according to the relevant national and international guidelines.
Insect sample preparation
A N. lugens colony was maintained on rice (Oryza sativa) variety Taichung Native 1 (TN1, a hopper susceptible variety) in a greenhouse of the China National Rice Research Institute under the conditions of 28 ± 1 °C, 80 ± 10% relative humidity and a 16 L/8D photoperiod34,46. Eggs, first- to fifth-instar nymphs (I1, I2, I3, I4 and I5), and newly emerged adults (female and male) (<24 h after molting) were collected and frozen at −80 °C until RNA extraction. Furthermore, a pooled sample including eggs, nymphs (I1-I5) and adults (male and female) was collected for PCR efficiency testing. For the insect samples of amino acid deprivation, newly formed third-instar nymphs were transferred to the standard artificial diet D-97 or modified artificial diets without one of the 20 amino acids46. For each diet type, 250 individuals were prepared in groups of 25 (a total of 10 groups). After four days on the diet, five individuals of each group were collected and pooled as one sample (a total of 50 individuals) and frozen at −80 °C until RNA extraction. This experiment was repeated three times as independent biological replicates.
To evaluate the effect of temperature, 180 newly emerged female adults (<24 h after molting) were placed into 18 glass tubes in a group of 10. Three of the tubes (replications) were exposed under one of the 6 temperature regimes (0 °C, 8 °C, 18 °C, 28 °C, 38 °C, 48 °C) for 2 h in an incubator (DC-3010, Jiangnan Equipment, Hangzhou, China). The nymphs then were recovered at 28 ± 1 °C for 2 h. The surviving individuals were frozen in liquid nitrogen and stored at −80 °C.
RNAi samples were prepared according to a previously reported protocol36 as the following: four dsRNAs were synthesized from EdePRTase (dsEdePRTase), ylsArg4 (dsylsArg4-1 and dsylsArg4-2), and the green fluorescent protein (dsGFP) as dsEdePRTase and dsylsArg4 had previous successes of knocking down the targeted genes evaluated using N. lugens reference genes NlRPS11 and NlRPS15 36. A total of 90 fourth-instar nymphs (1-day old) in three groups of 30 individuals each (as three replicates) were injected with the four dsRNAs (in 50 nL) (dsEdePRTase, 0.5 μg/μL; dsylsArg4, 1.5 μg/μL; and dsGFP, 1.5 μg/μL as negative controls, respectively) at the thorax between the mesocoxa and the hind coxa. Insects without dsRNA injection were used as blank control. Three days after the injection, the surviving individuals were collected and pooled for RT-qPCR analysis using the primers listed in Table 1. The experiment was repeated three times as independent biological replicates.
RNA extraction and cDNA synthesis
Total RNA was extracted from the collected samples using the Trizol Reagent (Invitrogen, Shanghai, China) and the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The RNA purity was measured with a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Rockford, USA) and the integrity was checked by agarose gel electrophoresis. One microgram total RNA was reverse-transcribed to cDNA by using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix kit (TransGen Biotech, Beijing, China).
Reference gene candidates from YLS and cloning
A total of eight housekeeping genes was selected from YLS draft genome sequences (Genbank accession numbers: JRMI00000000.1) as candidates of the most consistently expressed reference gene(s) to be used in RT-qPCR studies. They are γ-actin (ylsACT), α-tubulin (ylsTUB), ribosomal protein S11 (ylsRPS11), ribosomal protein S19A/S15e (ylsRPS15e), ribosomal protein S15P/S13E (ylsRPS15p), elongation factor 1α (ylsEF1α), glyceraldehyde-3-phosphate dehydrogenase (ylsGAPDH) and ubiquitin (ylsUBQ). These gene types have been used widely as reference genes in studies of insects and fungi. Primers were designed based on the sequences selected by the PrimerQuest software (Integrated DNA Technologies, Inc, Coralville, IA, USA) (Table 1). Additionally, BLAST analyses were performed to verify their specificity (Fig. 1). The PCR products of the female adults were sequenced by an ABI 3730 automated sequencer (Applied Biosystems, Foster City, CA, USA) in both directions. The obtained sequences were submitted to GenBank database (AB914563-AB914566).
RT-qPCR
Prior to the RT-qPCR amplification, a portion of cDNA was used to construct standard curves for PCR condition optimization and calculation of PCR efficiency of the eight selected reference gene candidates. For this purpose, five cDNA quantities (0.2, 2, 10, 50, 200 ng) were tested. PCR amplification efficiencies were calculated using the formula E = (10[−1/slope] − 1) × 100.
The RT-qPCR experiments were performed using the TransStart Top Green qPCR SuperMix- -UDG (TransGen Biotech) according to the manufacturer’s instructions on an ABI 7500 Real Time PCR System. Amplification reaction volume was 20 μL with 3 μL cDNA as template and a final concentration of 200 nM primers. The following thermal cycling condition was used: initial denaturation at 95 °C for 2 min, followed by 40 cycles of 95 °C for 20 s and 60 °C for 1 min. After all reactions, a melting curve analysis from 65 °C to 95 °C was applied to ensure consistency and specificity of the amplified product, and the most adequate annealing temperature was determined and used for PCR reaction. Each primer pair was checked for size specificity of the amplicon using 1.5% agarose gel electrophoresis and ethidium bromide staining. A reaction mix without template (no template control) was used to detect possible reagent contamination. The average of the Ct values was determined using manual quantification settings. The values of Ct for the control wells were excluded from further analysis, as these values were greater than 35 or not detectable.
Determination of expression stability
Stabilities of the reference gene candidates were evaluated by BestKeeper38, geNorm10, NormFinder47 and the ΔCt method11. BestKeeper analysis uses Ct values directly, while geNorm, NormFinder and ΔCt method use transformed Ct values of (1 + E)−ΔCt. In addition, RefFinder48, a web-based comprehensive platform that integrates the four above mentioned algorithms, was used for the overall ranking of the stability of these reference gene candidates. All data were analyzed according to the perspective instructions of the software used.
Validation of selected reference genes
Three YLS originated genes that are involved in amino acids biosynthetic pathways including NlylsSDH 34, EdePRTase 36 and ylsArg4 (unpublished data) were used as target genes to validate the selected YLS reference gene candidates using the 2−ΔΔCt method. Developmental stage and sex samples, samples of methionine- or histidine-free artificial diets, and RNAi samples were used.
Additional Information
How to cite this article: Wan, P.-J. et al. Reference genes for quantitative real-time PCR analysis in symbiont Entomomyces delphacidicola of Nilaparvata lugens (Stål). Sci. Rep. 7, 42206; doi: 10.1038/srep42206 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This research was supported by grants of the National Natural Science Foundation of China (31371939 and 31501637), the Zhejiang Provincial Natural Science Foundation of China (Q15C140014), and the Rice Pest Management Research Group of the Agricultural Science and Technology Innovation Program of China Academy of Agricultural Science.
Footnotes
The authors declare no competing financial interests.
Author Contributions P.J.W. and Q.F. conceived and designed the experiments; P.J.W., Y.H.T., S.Y.Y., J.C.H., W.X.W. and F.X.L. performed the experiments; P.J.W. analyzed the data; P.J.W. wrote the manuscript.
References
- Sun M., Lu M. X., Tang X. T. & Du Y. Z. Exploring valid reference genes for quantitative real-time PCR analysis in Sesamia inferens (Lepidoptera: Noctuidae). PLoS One 10, e0115979 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H. et al. Selection of reference genes for RT-qPCR analysis in the monarch butterfly, Danaus plexippus (L.), a migrating bio-indicator. PLoS ONE 10, e0129482 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schefe J. H., Lehmann K. E., Buschmann I. R., Unger T. & Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression’s CT difference” formula. J. Mol. Med. 84, 901–910 (2006). [DOI] [PubMed] [Google Scholar]
- Mahoney D. J. et al. Real-time RT-PCR analysis of housekeeping genes in human skeletal muscle following acute exercise. Physiol. Genomics 18, 226–231 (2004). [DOI] [PubMed] [Google Scholar]
- Thellin O. et al. Housekeeping genes as internal standards: use and limits. J. Biotechnol. 75, 291–295 (1999). [DOI] [PubMed] [Google Scholar]
- Lee P. D., Sladek R., Greenwood C. M. T. & Hudson T. J. Control genes and variability: Absence of ubiquitous reference transcripts in diverse mammalian expression studies. Genome Res. 12, 292–297 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin S. A. Developments in real-time PCR research and molecular diagnostics. Expert Rev. Mol. Diagn. 10, 713–715 (2010). [DOI] [PubMed] [Google Scholar]
- Butte A. J., Dzau V. J. & Glueck S. B. Further defining housekeeping, or “maintenance,” genes Focus on “A compendium of gene expression in normal human tissues”. Physiol. Genomics 7, 95–96 (2001). [DOI] [PubMed] [Google Scholar]
- Czechowski T., Stitt M., Altmann T., Udvardi M. K. & Scheible W. R. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139, 5–17 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, 1–12 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver N., Best S., Jiang J. & Thein S. L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 7 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcial-Quino J. et al. Validation of housekeeping genes as an internal control for gene expression studies in Giardia lamblia using quantitative real-time PCR. Gene 581, 21–30 (2016). [DOI] [PubMed] [Google Scholar]
- Klie M. & Debener T. Identification of superior reference genes for data normalisation of expression studies via quantitative PCR in hybrid roses (Rosa hybrida). BMC Res. Note 4, 1–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento C. S. et al. Identification of suitable reference genes for real time quantitative polymerase chain reaction assays on pectoralis major muscle in chicken (Gallus gallus). PLoS ONE 10, e0127935 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez L. et al. The lack of a systematic validation of reference genes: a serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol. J. 6, 609–618 (2008). [DOI] [PubMed] [Google Scholar]
- Bustin S. A. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55 (2009). [DOI] [PubMed] [Google Scholar]
- Pareek C. S., Smoczynski R. & Tretyn A. Sequencing technologies and genome sequencing. J. Appl. Genet. 52, 413–435 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk E. L., Auger H., Jaszczyszyn Y. & Thermes C. Ten years of next-generation sequencing technology. Trends Genet. 30, 418–426 (2014). [DOI] [PubMed] [Google Scholar]
- Mardis E. R. Next-generation sequencing platforms. Annu. Rev. Anal. Chem. 6, 287–303 (2013). [DOI] [PubMed] [Google Scholar]
- Lu S. et al. Probing meiotic recombination and aneuploidy of single sperm cells by whole-genome sequencing. Science 338, 1627–1630 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H.-W. et al. Genomic analysis of an Ascomycete fungus from the rice planthopper reveals how it adapts to an endosymbiotic lifestyle. Genome Biol. Evol. 7, 2623–2634 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Cheng L. L. & Hou R. F. Studies on the intracellular yeast-like symbiote in the brown planthopper, Nilaparvata lugens Stål. J. Appl. Entomol. 92, 440–449 (1981). [Google Scholar]
- Dong S. Z., Pang K., Bai X., Yu X. P. & Hao P. Y. Identification of two species of yeast-like symbiotes in the brown planthopper, Nilaparvata lugens. Curr. Microbiol. 62, 1133–1138 (2011). [DOI] [PubMed] [Google Scholar]
- Pang K. et al. Cultivation, identification and quantification of one species of yeast-like symbiotes, Candida, in the rice brown planthopper, Nilaparvata lugens. Insect Sci. 19, 477–484 (2012). [Google Scholar]
- Xue J. et al. Genomes of the rice pest brown planthopper and its endosymbionts reveal complex complementary contributions for host adaptation. Genome Biol. 15, 521 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y.-Y. et al. Genomic insights into the serine protease gene family and expression profile analysis in the planthopper, Nilaparvata lugens. BMC Genomics 15, 507 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.-J. et al. Two insulin receptors determine alternative wing morphs in planthoppers. Nature 519, 464–467 (2015). [DOI] [PubMed] [Google Scholar]
- Huang H.-J. et al. A salivary sheath protein essential for the interaction of the brown planthopper with rice plants. Insect Biochem. Mol. Biol. 66, 77–87 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang B.-X. et al. Bicaudal-C plays a vital role in oogenesis in Nilaparvata lugens (Hemiptera: Delphacidae). J. Insect Physiol. 79, 19–26 (2015). [DOI] [PubMed] [Google Scholar]
- Ge L.-Q. et al. Silencing a sugar transporter gene reduces growth and fecundity in the brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Sci. Rep. 5, 12194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Ding Z., Zhang C., Yang B. & Liu Z. Gene knockdown by intro-thoracic injection of double-stranded RNA in the brown planthopper, Nilaparvata lugens. Insect Biochem. Mol. Biol. 40, 666–671 (2010). [DOI] [PubMed] [Google Scholar]
- Li H., Guan R., Guo H. & Miao X. New insights into an RNAi approach for plant defence against piercing-sucking and stem-borer insect pests. Plant, Cell Environ. 38, 2277–2285 (2015). [DOI] [PubMed] [Google Scholar]
- Yu R. et al. The insect ecdysone receptor is a good potential target for RNAi-based pest control. Int. J. Biol. Sci. 10, 1171–1180 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan P.-J. et al. RNA interference-aided knockdown of a putative saccharopine dehydrogenase leads to abnormal ecdysis in the brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Bull. Entomol. Res. 105, 390–398 (2015). [DOI] [PubMed] [Google Scholar]
- Wan P.-J. et al. Pathways of amino acid degradation in Nilaparvata lugens (Stål) with special reference to lysine-ketoglutarate reductase/saccharopine dehydrogenase (LKR/SDH). PLoS ONE 10, e0127789 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan P.-J. et al. ATP phosphoribosyltransferase from symbiont Entomomyces delphacidicola invovled in histidine biosynthesis of Nilaparvata lugens (Stål). Amino Acids, 1–13 (2016). [DOI] [PubMed] [Google Scholar]
- Bustin & Stephen A. A-Z of quantitative PCR. (International University Line, 2004). [Google Scholar]
- Pfaffl M. W., Tichopad A., Prgomet C. & Neuvians T. P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper – Excel-based tool using pair-wise correlations. Biotechnol. Lett. 26 (2004). [DOI] [PubMed] [Google Scholar]
- Wan P.-J. et al. Constructing the major biosynthesis pathways for amino acids in the brown planthopper, Nilaparvata lugens Stål (Hemiptera: Delphacidae), based on the transcriptome data. Insect Mol. Biol. 23, 152–164 (2014). [DOI] [PubMed] [Google Scholar]
- Zhu X. et al. Selection and evaluation of reference genes for expression analysis using qRT-PCR in the beet armyworm Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae). PLoS ONE 9, e84730 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M. et al. Selection and evaluation of potential reference genes for gene expression analysis in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) using reverse-transcription quantitative PCR. PLoS One 9, e86503 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge H. J. M. et al. Evidence based selection of housekeeping genes. PLoS ONE 2, e898 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozera B. & Rapacz M. Reference genes in real-time PCR. J. Appl. Genet. 54 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M. R. et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. 7, 665–674 (2005). [DOI] [PubMed] [Google Scholar]
- Radonic A. et al. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 313, 856–862 (2004). [DOI] [PubMed] [Google Scholar]
- Fu Q., Zhang Z., Hu C., Lai F. & Sun Z. A chemically defined diet enables continuous rearing of the brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae). Appl. Entomol. Zool. 36, 111–116 (2001). [Google Scholar]
- Andersen C. L., Jensen J. L. & Orntoft T. F. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 64, 5245–5250 (2004). [DOI] [PubMed] [Google Scholar]
- Xie F., Xiao P., Chen D., Xu L. & Zhang B. miRDeepFinder: a miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. (2012). [DOI] [PubMed] [Google Scholar]