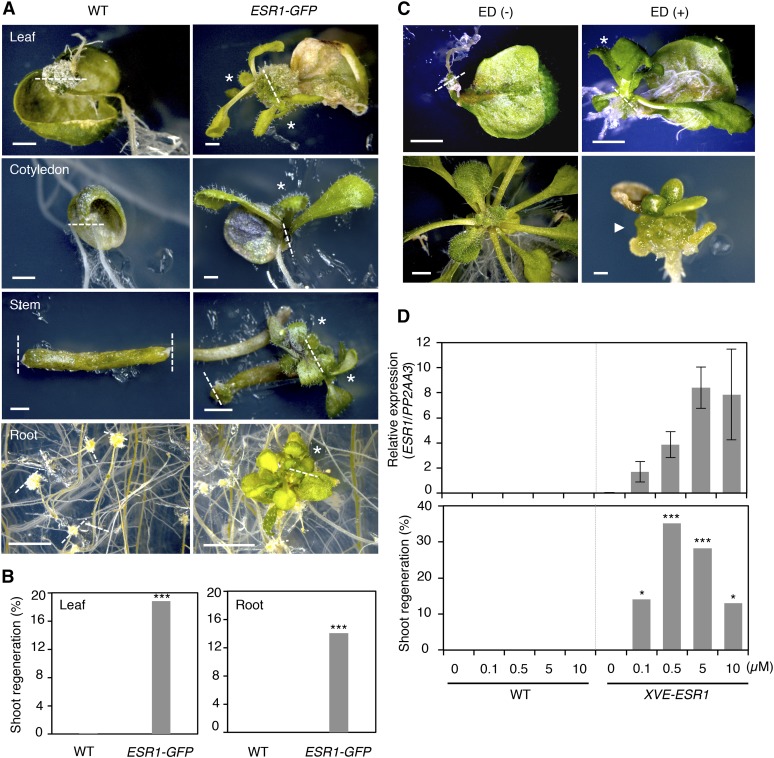
Figure 5.
ESR1 Promotes Shoot Regeneration at Wound Sites.
(A) Induction of shoot regeneration at wound sites of ESR1-GFP explants. Wild-type and ESR1-GFP explants were cultured on phytohormone-free MS medium for 50 d (leaves), 30 d (cotyledons and inflorescence stems), and 40 d (roots). Dashed lines represent wound sites and asterisks mark regenerating shoots.
(B) Quantitative analysis of shoot regeneration at wound sites. Leaf and root explants were cultured on phytohormone-free MS medium for 55 d. Data are shown as frequency (%) of explants regenerating shoots (n = 646 for wild-type leaves, 170 for ESR1-GFP leaves, 576 for wild-type roots, and 634 for ESR1-GFP roots). Statistical significance was determined by a proportion test (***P < 0.001).
(C) Induction of shoot regeneration at wound sites of XVE-ESR1 leaf explants. Top panel: XVE-ESR1 leaf explants were cultured on phytohormone-free MS medium in the absence (–) or presence (+) of 10 µM 17β-estradiol (ED). Bottom panel: Unwounded XVE-ESR1 plants develop callus in the presence of 10 µM ED. The dashed line represents wound sites and an asterisk marks regenerating shoots. An arrowhead marks callus developing from hypocotyls in intact XVE-ESR1 plants. Bars = 1 mm in (A) and (C).
(D) The frequency of shoot regeneration positively correlates with the level of ESR1 expression in XVE-ESR1 plants. ESR1 expression and shoot regeneration were quantified at 6 and 16 d, respectively, after the application of 0.1 to 10 μM β-estradiol. Expression levels are normalized against those of PP2AA3. Expression data are mean ± se (n = 3, biological replicates). Shoot regeneration is quantified as the frequency (%) of explants regenerating shoots (n = 50 per β-estradiol concentration). Statistical significance was determined by a proportion test (***P < 0.001 and *P < 0.1).