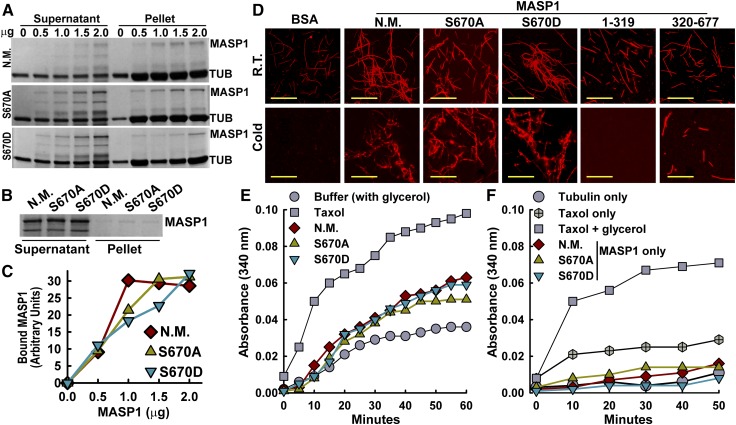
Figure 6.
MASP1 MT Interaction and MT Bundling.
(A) Cosedimentation MT binding assays using 0 to 2.0 mg of unmutated MASP1 (N.M.), MASP1S670A (phosphonull), or MASP1S670D (phosphomimic). TUB, tubulin. Note that the addition of even a small amount of MASP1 increased the amount of tubulin in the pellet fraction (to preserve MASP1 solubility, the final sedimentation was done at 10°C, which partially destabilized microtubules). Images shown are Coomassie-stained gels and are representative of two to three experiments.
(B) Minus MT control for the MASP1 MT binding experiments is shown in (A). Negligible MASP1 precipitation was seen in the absence of MTs. Gel shown is typical of several repeated experiments.
(C) Quantification of MASP1 sedimentation with microtubules. Data are means from two experiments.
(D) MASP1 MT bundling and stabilization in vitro visualized using rhodamine-labeled MTs. R.T., room temperature incubation of MTs with the indicated MASP1 alleles or truncations or BSA. Cold = 10°C, 45 min. Bars = 10 μm.
(E) Turbidity assay measuring MT polymerization in the presence of taxol, unmutated MASP1 (N.M.), MASP1S670A (phosphonull), or MASP1S670D (phosphomimic). Each type of MASP1 protein was present at 10 μM. All assays included glycerol to promote MT polymerization. Data shown are representative of three independent experiments.
(F) Turbidity assay performed in the same manner as in (E) except that glycerol was omitted for all reactions (except taxol + glycerol) to test whether MASP1 could promote MT polymerization.