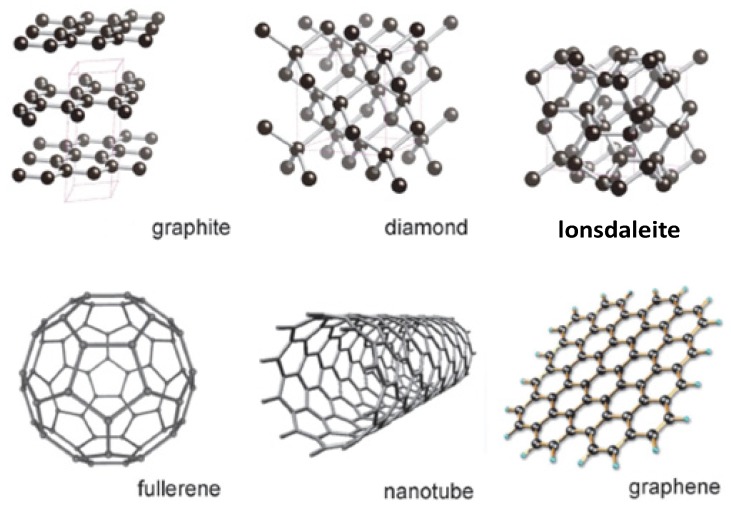
Figure 2.
The common naturally-occurring sp2 and sp3 allotropes of carbon occur in different crystallographic forms. Graphite: Hexagonal; stacked flat layers of 3-coordinated sp2 C. Diamond: Cubic; framework of 4-coordinated sp3 C. Lonsdaleite: Hexagonal; framework of 4-coordinated sp3 C. Fullerenes: Closed cage molecules sp2 C: C60, C70, C76, etc. Nanotubes cylindrical fibers of sp2 C, single tubes or nested. Graphene: one-atom-thick graphitic layers with sp2 bonding. Reprinted with permission from [3]. Copyright 2013 Mineralogical Society of America.