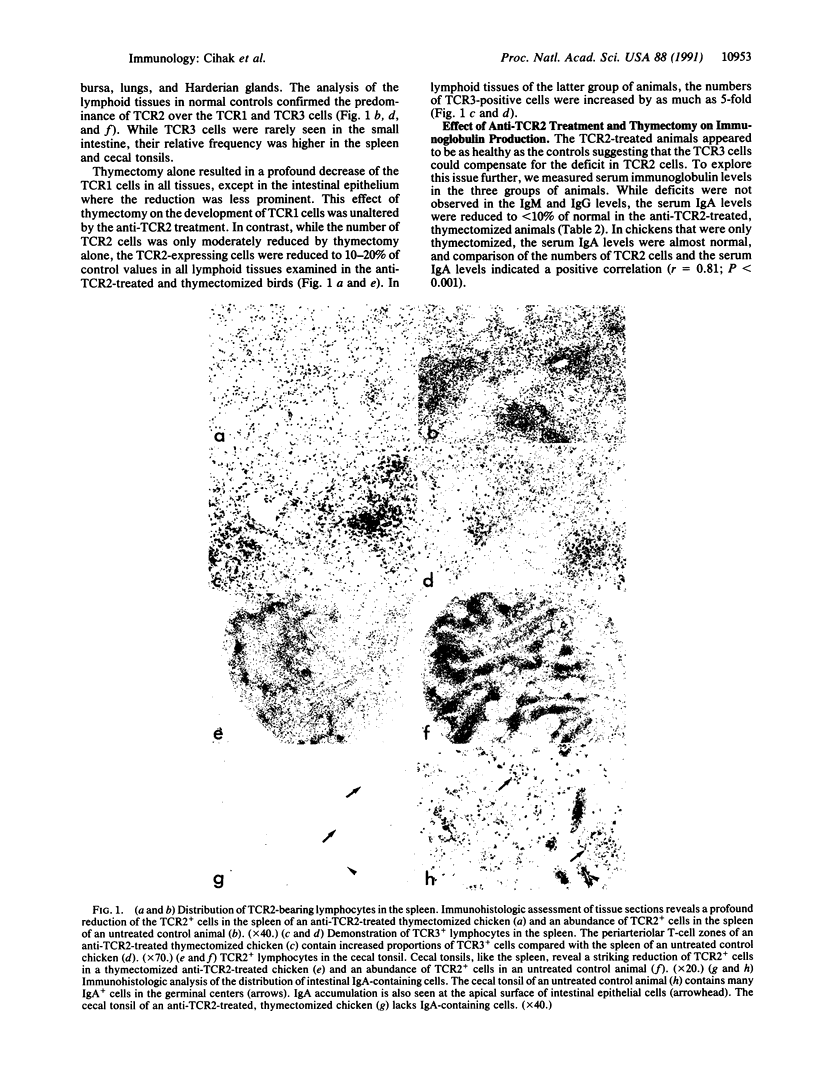
Abstract
While alpha beta T cells in mammals may express one of many variable (V) gene families in the beta locus, chickens have only two V beta gene families. The avian V beta 2+ T cells are recognized by the T-cell receptor 3 (TCR3) monoclonal antibody and V beta 1+ T cells are recognized by the TCR2 antibody, which we used to selectively suppress development of V beta 1+ T cells in order to examine their functional role. Suppression was accomplished by multiple injections of anti-TCR2 antibodies beginning in embryonic life and perpetuated by thymectomy 8 days after hatching. Young birds thus depleted of V beta 1+ T cells had greater than normal numbers of V beta 2+ T cells and appeared as healthy as thymectomized and untreated controls. While production of IgM and IgG antibodies was unimpaired, IgA antibody production was severely compromised in the V beta 1-depleted birds. The levels of secretory IgA in bile and lung lavage fluid were reduced 1000- to 10,000-fold and secretory IgA antibodies were not produced in response to mucosal immunization. B-cell production of IgA antibodies thus appears to require T cells expressing the V beta 1 genes, whereas T cells that express the V beta 2 genes lack this capacity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson E. B., Strober W. Regulation of IgA secretion by T cell clones derived from the human gastrointestinal tract. J Immunol. 1988 Mar 15;140(6):1874–1882. [PubMed] [Google Scholar]
- COOPER M. D., PETERSON R. D., GOOD R. A. DELINEATION OF THE THYMIC AND BURSAL LYMPHOID SYSTEMS IN THE CHICKEN. Nature. 1965 Jan 9;205:143–146. doi: 10.1038/205143a0. [DOI] [PubMed] [Google Scholar]
- Char D., Sanchez P., Chen C. L., Bucy R. P., Cooper M. D. A third sublineage of avian T cells can be identified with a T cell receptor-3-specific antibody. J Immunol. 1990 Dec 1;145(11):3547–3555. [PubMed] [Google Scholar]
- Chen C. H., Sowder J. T., Lahti J. M., Cihak J., Lösch U., Cooper M. D. TCR3: a third T-cell receptor in the chicken. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2351–2355. doi: 10.1073/pnas.86.7.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Ager L. L., Gartland G. L., Cooper M. D. Identification of a T3/T cell receptor complex in chickens. J Exp Med. 1986 Jul 1;164(1):375–380. doi: 10.1084/jem.164.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Cihak J., Lösch U., Cooper M. D. Differential expression of two T cell receptors, TcR1 and TcR2, on chicken lymphocytes. Eur J Immunol. 1988 Apr;18(4):539–543. doi: 10.1002/eji.1830180408. [DOI] [PubMed] [Google Scholar]
- Cihak J., Ziegler-Heitbrock H. W., Trainer H., Schranner I., Merkenschlager M., Lösch U. Characterization and functional properties of a novel monoclonal antibody which identifies a T cell receptor in chickens. Eur J Immunol. 1988 Apr;18(4):533–537. doi: 10.1002/eji.1830180407. [DOI] [PubMed] [Google Scholar]
- Clough J. D., Mims L. H., Strober W. Deficient IgA antibody responses to arsanilic acid bovine serum albumin (BSA) in neonatally thymectomized rabbits. J Immunol. 1971 Jun;106(6):1624–1629. [PubMed] [Google Scholar]
- Crewther P., Warner N. L. Serum immunoglobulins and antibodies in congenitally athymic (nude) mice. Aust J Exp Biol Med Sci. 1972 Oct;50(5):625–635. doi: 10.1038/icb.1972.55. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Kawanishi H., Saltzman L. E., Strober W. Mechanisms regulating IgA class-specific immunoglobulin production in murine gut-associated lymphoid tissues. I. T cells derived from Peyer's patches that switch sIgM B cells to sIgA B cells in vitro. J Exp Med. 1983 Feb 1;157(2):433–450. doi: 10.1084/jem.157.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. H., Kagnoff M. F. Transforming growth factor-beta 1 is a costimulator for IgA production. J Immunol. 1990 May 1;144(9):3411–3416. [PubMed] [Google Scholar]
- Kincade P. W., Cooper M. D. Immunoglobulin A: site and sequence of expression in developing chicks. Science. 1973 Jan 26;179(4071):398–400. doi: 10.1126/science.179.4071.398. [DOI] [PubMed] [Google Scholar]
- Kincade P. W., Lawton A. R., Bockman D. E., Cooper M. D. Suppression of immunoglobulin G synthesis as a result of antibody-mediated suppression of immunoglobulin M synthesis in chickens. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1918–1925. doi: 10.1073/pnas.67.4.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono H., Cooper M. D., Kearney J. F., Mosteller L. M., Michalek S. M., Koopman W. J., McGhee J. R. Isotype specificity of helper T cell clones. Peyer's patch Th cells preferentially collaborate with mature IgA B cells for IgA responses. J Exp Med. 1984 Mar 1;159(3):798–811. doi: 10.1084/jem.159.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimoto D. Y., Harriman G. R., Strober W. Regulation of IgA differentiation in CH12LX B cells by lymphokines. IL-4 induces membrane IgM-positive CH12LX cells to express membrane IgA and IL-5 induces membrane IgA-positive CH12LX cells to secrete IgA. J Immunol. 1988 Aug 1;141(3):713–720. [PubMed] [Google Scholar]
- Lahti J. M., Chen C. L., Tjoelker L. W., Pickel J. M., Schat K. A., Calnek B. W., Thompson C. B., Cooper M. D. Two distinct alpha beta T-cell lineages can be distinguished by the differential usage of T-cell receptor V beta gene segments. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10956–10960. doi: 10.1073/pnas.88.23.10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Elson C. O., Kiyono H. Regulation of IgA synthesis and immune response by T cells and interleukins. J Clin Immunol. 1989 May;9(3):175–199. doi: 10.1007/BF00916814. [DOI] [PubMed] [Google Scholar]
- Perey D. Y., Biennenstock J. Effects of bursectomy and thymectomy on ontogeny of fowl IgA, IgG, and IgM. J Immunol. 1973 Aug;111(2):633–637. [PubMed] [Google Scholar]
- Peterson R. D., Cooper M. D., Good R. A. Lymphoid tissue abnormalities associated with ataxia-telangiectasia. Am J Med. 1966 Sep;41(3):342–359. doi: 10.1016/0002-9343(66)90080-5. [DOI] [PubMed] [Google Scholar]
- Pritchard H., Riddaway J., Micklem H. S. Immune responses in congenitally thymus-less mice. II. Quantitative studies of serum immunoglobulins, the antibody response to sheep erythrocytes, and the effect of thymus allografting. Clin Exp Immunol. 1973 Jan;13(1):125–138. [PMC free article] [PubMed] [Google Scholar]
- Schiffer M., Wu T. T., Kabat E. A. Subgroups of variable region genes of beta chains of T-cell receptors for antigen. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4461–4463. doi: 10.1073/pnas.83.12.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowder J. T., Chen C. L., Ager L. L., Chan M. M., Cooper M. D. A large subpopulation of avian T cells express a homologue of the mammalian T gamma/delta receptor. J Exp Med. 1988 Feb 1;167(2):315–322. doi: 10.1084/jem.167.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjoelker L. W., Carlson L. M., Lee K., Lahti J., McCormack W. T., Leiden J. M., Chen C. L., Cooper M. D., Thompson C. B. Evolutionary conservation of antigen recognition: the chicken T-cell receptor beta chain. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7856–7860. doi: 10.1073/pnas.87.20.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]