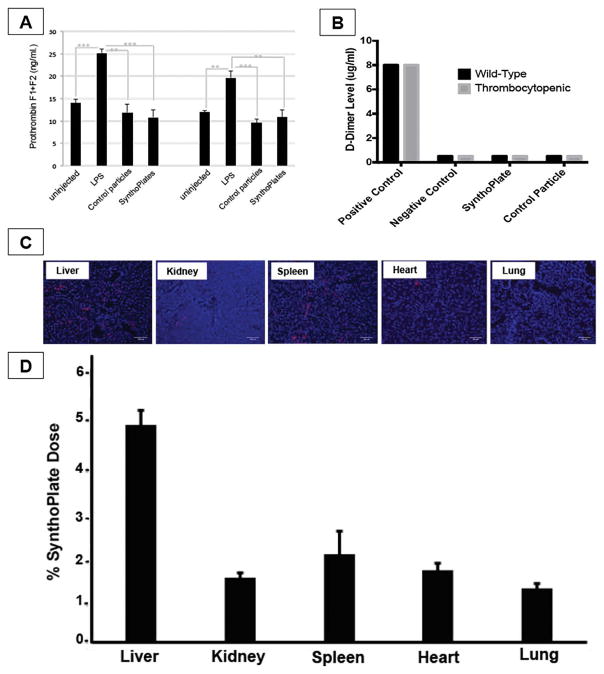
Figure 6.
[A] Prothrombin F1 + F2 fragment ELISA assay, as well as, [B] Stago D-dimer assay in mice injected with SynthoPlateTM indicate that SynthoPlateTM vesicles do not trigger spontaneous formation of thrombin and fibrin in plasma (minimal systemic pro-coagulant risk); [C] Representative fluorescent images (blue: DAPI-stained nuclei, red: Rh-B labeled particles) of harvested tissue cryo-sections and [D] histogram showing systemic localization of SynthoPlateTM vesicles in TCP mice at the 2 hr circulation period indicate that ~15% of injected dose is cleared during the 2 hr period, with liver and spleen being the principal organs of vesicle clearance, with much reduced clearance in kidney, heart and lungs.