Abstract
The Baja California Peninsula in México has about 670 species of macroalgae along its coast. Species richness increases the probability of finding native macroalgae with potential as sources of bioactive compounds suitable for health, pharmacological, and cosmetic ingredients. To understand the biotechnological value of macroalgae from the peninsula, ethanol extracts from 17 macroalgae (four Chlorophyta, six Rhodophyta, seven Ochrophyta) were screened for antioxidant potential. To determine the antioxidant capacity of macroalgal extracts, 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power, and nitric oxide radical scavenging as well as total phenolic content (TPC) were measured. Extracts of the brown macroalgae were most active. Among these, Eisenia arborea, Padina concrecens, and Cystoseira osmundacea had the highest TPC and exhibited the strongest radical scavenging activities. Correlations were found between TPC macroalgal and scavenging capacity, indicating an important role of polyphenols as antioxidants. This suggests that some brown macroalgae from Baja California Peninsula may be a good source of natural bioactive compounds.
Keywords: Macroalgae, Ethanol extracts, Antioxidant activity, Phenolic content
Introduction
Marine organisms are rich sources of structurally diverse bioactive compounds with valuable nutraceutical, pharmaceutical, and cosmeceutical potential (Hafting et al. 2015). In recent years, many novel bioactive compounds have been isolated from macroalgae, especially natural antioxidants capable of inhibiting reactive oxygen radical mediated oxidative stress (Kalaiselvan et al. 2016; Custódio et al. 2016). Red (Rhodophyta), green (Chlorophyta), and brown (Phaeophyta) macroalgae offer a wide variety of natural compounds with interesting properties as natural antioxidants (Andrade et al. 2013; Osuna-Ruiz et al. 2016). Macroalgae have antioxidant systems to counteract environmental stresses, and produce bioactive compounds, including polyphenols, alkaloids, terpenes, phycocyanins, carotenoids, and various enzymes (Maharana et al. 2015). The polyphenols from macroalgae are more potent that their analogues from terrestrial plants (Nagayama et al. 2002), and possess strong antioxidant activity based on up to eight interconnected phenol rings (Fernando et al. 2016). In the last years, the searching has been focused on natural antioxidants capable of inhibiting reactive oxygen radical mediated oxidative stress (Machu et al. 2015; Vizetto-Duarte et al. 2016).
Few published studies have explored the biological activity of macroalgae from Baja California, México, which include anticoagulant, antimicrobial, antifouling, and cytotoxicity properties (Muñoz-Ochoa et al. 2009, 2010; Águila-Ramírez et al. 2012). Marine algae are one of the important renewable resources of the Baja California Peninsula. Of the brown macroalgae, Macrocystis pyrifera is harvested, processed, and used for aquaculture purposes. Total natural production of M. pyrifera is about 100,000 t per year (Hernández-Carmona et al. 1991) and only 1% of this is used by humans. However, there are other abundant species (e.g. Eisenia arborea, Sargassum horridum, Gelidium robustum, Codium spp., and Acanthopora spicifera) that have economic and biotechnological potential. Although the antioxidant activity of numerous genera of algae has been reported from North Borneo (Matanjun et al. 2008) and the Hawaian Islands (Kelman et al. 2012), among other regions (Raja et al. 2015), this is the first time that marine macroalgae from Baja California Peninsula were assayed for antioxidant activity. This study was conducted to quantify the antioxidant activity of extracts from 17 macroalgal species along the coast of the Baja California Peninsula.
Material and methods
Macroalgae samples included four Chlorophyta, six Rhodophyta, and seven Ochrophyta (phaeophyta) (Table 1). Samples were collected in along the west coast of the Baja California Peninsula, Mexico (25°50′46′′N, 111°58′22′′O) from August 2004 through July 2013 as described in Table 1. Samples were rinsed with tap water to remove salts, sand and epiphytes, and sun-dried (Muñoz-Ochoa et al. 2010). The dried material was milled using a blender and stored at −80 °C. Samples were identified to genus and species level by three phycologists using taxonomic keys for future reference.
Table 1.
Location and date of collection of macroalgal species from the west coast of the Baja California Peninsula, used in this study for their antioxidant activities and phenolic content
| Species | Collection location | Collection date | |
|---|---|---|---|
| Chlorophyta | |||
| Codium amplivesiculatum | Setchell & N.L. Gardner 1924 | Tarabillas | 13, July, 2013 |
| Codium simulans | Setchell & N.L. Gardner 1924 | Margarita Island | 29, Juny, 2006 |
| Cladophora spp. | Cerritos | 9, May, 2006 | |
| Ulva dactylifera | Setchell & N.L. Gardner 1920 | Cerritos | 9, May, 2006 |
| Rhodophyta | |||
| Acanthophora spicifera | (M. Vahl) Børgesen 1910 | Costa Baja | 27, April, 2007 |
| Amphiroa valonioides | Yendo 1902 | Los Cerritos, Todos Santos | 16, March, 2007 |
| Gelidium robustum | (N.L. Gardner) Hollenberg & I.A. Abbott 1965 | Punta Eugenia | 9, August, 2006 |
| Neorhodomela larix | (Turner) Masuda 1982 | Punta Eugenia | 9, August, 2006 |
| Rodhymenia californica | Kylin 1931 | Punta Eugenia | 9, August, 2006 |
| Ochrophyta | |||
| Cystoseira osmundacea | (Turner) C. Agardh 1820 | Puerto Clam Bay | 9, August, 2006 |
| Dictyopteris delicatula | J.V. Lamouroux 1809 | Chester | 10, August, 2006 |
| Eisenia arborea | Areschoug 1876 | Punta Eugenia | 9, August, 2006 |
| Hydroclathratus clathratus | (C. Agardh) M. Howe in N.L. Britton & C.F. Millspaugh 1920 | La Concha | 19, May, 2006 |
| Macrocystis pyrifera | (Linnaeus) C. Agardh 1820 | Punta Eugenia | 9, August, 2006 |
| Neorhodomela larix | (Turner) Masuda 1982 | Chester | 10, August, 2006 |
| Padina concrecens | Thivy in W.R. Taylor 1945 | Punta Eugenia | 9, August, 2006 |
| Rosenvigea intricata | (J. Agardh) Børgesen 1914 | Gaviota Island | 12, August, 2004 |
| Sargassum horridium | Setchell & N.L. Gardner 1924 | La Concha | 15, May, 2006 |
Preparation of crude macroalgal extracts
The milled material was soaked in ethanol for eight at days 25 °C under gentle agitation (Muñoz-Ochoa et al. 2010). Then, ethanol was filtered and replaced (3 × 600 mL). Extracts were filtered through Whatman no.4 filter paper, and solvent removed using a rotatory vaccum evaporator to yield dry material. For antioxidant activity assays, a sample of each extract was prepared to provide a final test concentration of 10 mg mL−1 in ethanol. All extract samples were stored at 4 °C in a refrigerator until used.
In vitro antioxidant activity
1,1-diphenyl-2-picryl-hydrazil (DPPH) radical scavenging activity
Free radical scavenging capacity of the extracts was analyzed using a modified microplate method of the DPPH assay method (Brand-Williams et al. 1995). Different concentrations of extracts (25–400 µg mL−1) were mixed with a methanol solution containing DPPH radical (0.1 mM) freshly prepared. The mixtures were placed in the dark for 30 min at 25 °C; and absorbance was measured at 515 nm. The scavenging activity was calculated as:
Radical scavenging activity (%) = (A0 – A/A0) × 100, where A0 is the absorbance of the control and A is the absorbance of the sample.
A curve of extract concentration versus % DPPH was generated to estimate the concentration of extract needed to cause a 50% reduction of the initial DPPH concentration. This value is known as EC50 (efficient concentration or oxidation index) and is expressed in µg mL−1.
Nitric oxide scavenging activity assay
Nitric oxide was generated from sodium nitroprusside (SNP) dehydrate and measured by the Griess reagent (Andrade et al. 2013). 10 mM SNP in phosphate buffer was mixed with different concentrations of macroalgal ethanol extracts and incubated at 25 °C for 1 h. The extract at different concentrations (25–400 µg mL−1) was mixed with an equal volume of Griess reagent and absorbance was read at 562 nm. The same reaction mixture without the macroalgal extract but with the equivalent amount of ethanol served as the control. The percentage nitrite radical scavenging activity of the ethanol extracts were calculated using the following formula:
Nitric oxide scavenged (%) = (A0 – A/A0) × 100, where A0 is the absorbance of the control and A is the absorbance of the sample of extracts.
Ferric reducing antioxidant power (FRAP) assay
Antioxidant activity of algal extracts was determined by FRAP assay with slight modifications (Benzie and Strain 1996). Briefly, the FRAP reagent was made by mixing 300 mM acetate buffer (pH 3.6), 10 mM 2,4,6-tripyridyl-S-triazine (TPTZ) solution, and 20 mM FeCl3·6H2O in a 10:1:1 ratio and heated to 37 °C prior to use. The TPTZ solution was prepared by mixing equal volumes of 10 mM TPTZ with 40 mM HCl.
The FRAP reagent was added to each well of a 96-well microtiter plate. A blank reading was taken at 595 nm using a microplate reader (#550, Bio-Rad Laboratories, Hercules, CA). To each well, 20 μL of sample in triplicate was added, incubated for 8 min at 25 °C, and read at 595 nm. Triplicate standards of known Fe(II) concentrations were run simultaneously, using concentrations between 50 and 1000 μM FeSO4·7H2O as standard curve.
Determination of total phenolic compounds
The Folin–Ciocalteu colorimetric assay was used to quantify total polyphenol concentration in macroalgal samples (Singleton and Rossi 1965). The Folin–Ciocalteu reagent and the sample were mixed thoroughly. After 1 min, Na2CO3 was added, and the solution was thoroughly mixed. After incubation for 1 h at 25 °C, absorbance at 750 nm was recorded in the microplate reader and compared to a gallic acid calibration curve (0–1000 µg mL−1). The results are expressed as gallic acid equivalents (GAE) in mg 100 g−1 FW.
Statistical analysis
Values expressed are means of three replicate determinations ±SD. All statistical analyses were carried out using Prism 5.0 (GraphPad Software, La Jolla, CA). The data were tested for normality (Shapiro–Wilk test) and homogeneity of variances using the Leven test. To determine whether there were any differences among activities of samples, variance analysis (ANOVA) and Turkey post hoc test (p < 0.05, α = 0.05) were applied to the results. Correlations among data were calculated using Pearson’s correlation coefficient.
Results
Antioxidant activity DPPH scavenging activity
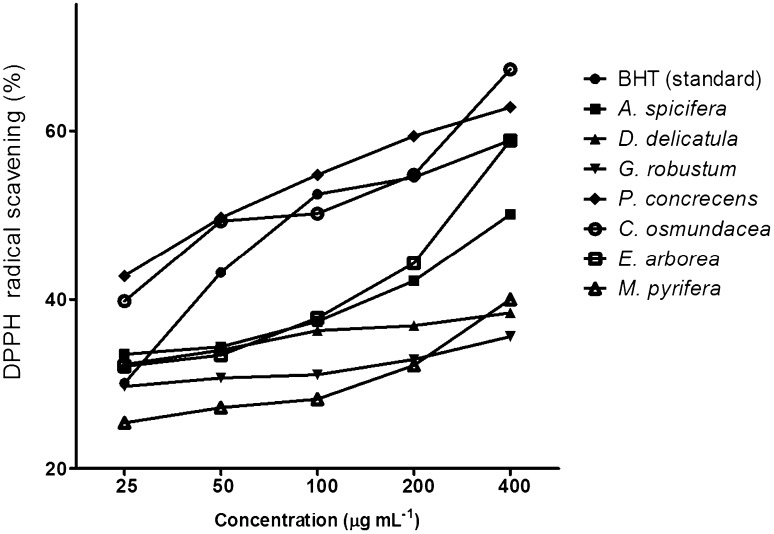
DPPH scavenging activity was significantly different between macroalgae species [(F (16, 34) = 201.5, p = 0.0001]. DPPH radical-scavenging activity at concentration of 400 µg mL−1 of the ethanol extract from the brown macroalga tested Cystoseira osmundacea was the most active (67.9%), compared to the other macroalgae. Other high activity was Padina concrecens (62.8%), Eisenia arborea (58.8%), and Acanthophora spicifera (50.4%). The other macroalgae had activity below 50%. Concentration dependency of antioxidant activity also was investigated in the 17 extracts. Seven were able to scavenge DPPH in a dose-dependant manner. The extract of P. concrecens, E. arborea and C. osmundacea were the most potent scavengers, similar or superior to butylated hydroxytoluene (BHT) used as the standard antioxidant in this study (58.9%) (Fig. 1).
Fig. 1.
DPPH radical scavenging activity (%) of macroalgal ethanol extracts at different concentrations (25, 50, 100, 200 and 400 µg mL) expressed as inhibition percentage
Almost all extracts showed antioxidant activity in varying degrees. Lower EC50 concentrations indicate higher antioxidant activity. In comparison to the EC50 of BHT (86.7 µg mL−1), P. concrecens and C. osmundacea had a relatively high antioxidant activity or low EC50: 50 and 69 µg mL−1, respectively (p < 0.05; Table 2). For Acanthophora spicifera, Gelidium robustum, and Dictyopteris delicatula, we were only able to calculate the EC35 (20.3, 355.2, and 72.9 µg mL−1, respectively) because in extract concentrations higher than 100 µg mL−1, the scavenging capacity decreased or did not change.
Table 2.
Antiradical capacity of macroalgal ethanol crude extracts, and the standard BHT
| Species | Radical | |
|---|---|---|
| DPPH | NO | |
| Butylated hydroxytoluene | 86.7c | – |
| Chlorophyta | ||
| U. dactylifera | – | 98.8c |
| Rhodophyta | ||
| A. spicifera | 20.3b | 370.1c |
| A. valonioides | – | 271.1c |
| G. robustum | 355.2b | – |
| N. larix | 305.8c | |
| R. californica | 105.2b | |
| Ochrophyta | ||
| H. clathratus | – | 316.8c |
| M. pyrifera | 227.2a | – |
| D. delicatula | 71.9b | 264.9c |
| C. osmundacea | 69.0c | 281.8c |
| E. arborea | 277.9c | 39.7c |
| P. concrecens | 50.0c | 129.8c |
EC values were expressed as µg mL−1
Efficient concentration (EC) values for DPPH scavenging activities (µg mL−1) and Nitric Oxid (NO) scavenging activities (µg mL−1). EC values were determinate from the linear regression curve of scavenging activities against the different concentration of algal extracts. EC value is defined as the amount of antioxidant necessary to decrease the initial DPPH/NO radicals concentration by 25, 35 and 50% respectively
aEC25 values (µg mL−1)
bEC35 values (µgmL−1)
cEC50 values (µg mL−1)
Nitric oxide scavenging activity assay
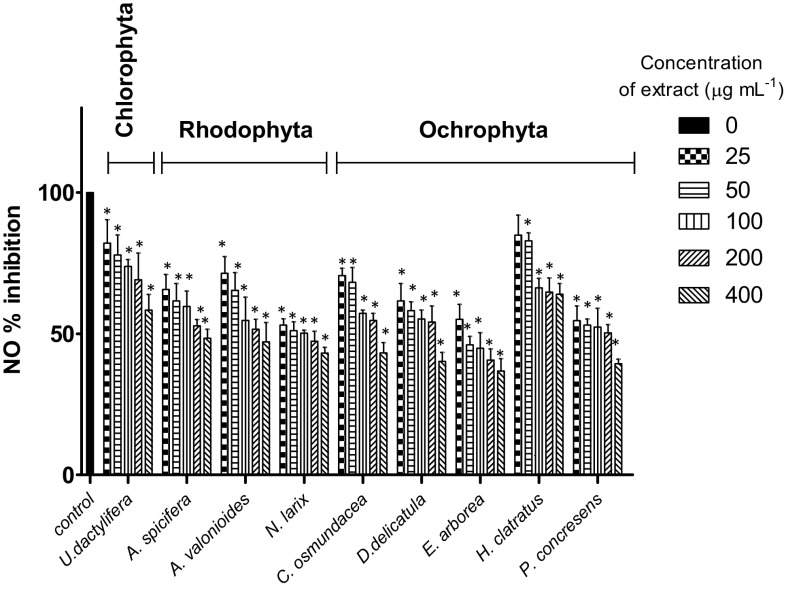
Nitric oxide scavenging activity was significantly different among macroalgae species [(F (8, 32) = 4.9, p = 0.0007]. Scavenging activity in 400 µg mL−1 ethanol extracts showed that the brown macroalgae E. arborea, P. concrecens, and D. delicatula had the highest capacity (p < 0.05), leading to reductions of 63.2, 60.6, and 59.8%, respectively, when compared with the control (Fig. 2). Concentration dependency of antioxidant activity was also measured. Nine extracts were able to scavenge >50% of the nitric oxide in a dose-dependent manner (Fig. 2). Highest scavenging activity, with relatively low EC50, occurred in E. arborea (39.7 µg mL−1) and Ulva dactylifera (98.7 µg mL−1).
Fig. 2.
Nitric Oxide scavenging activity (%) of macroalgal ethanol extracts at different concentrations (25, 50, 100, 200 and 400 µg mL) expressed as inhibition percentage. Asterisk indicates significant differences (p < 0.05) compared to the control
Ferric reducing antioxidant power (FRAP) assay
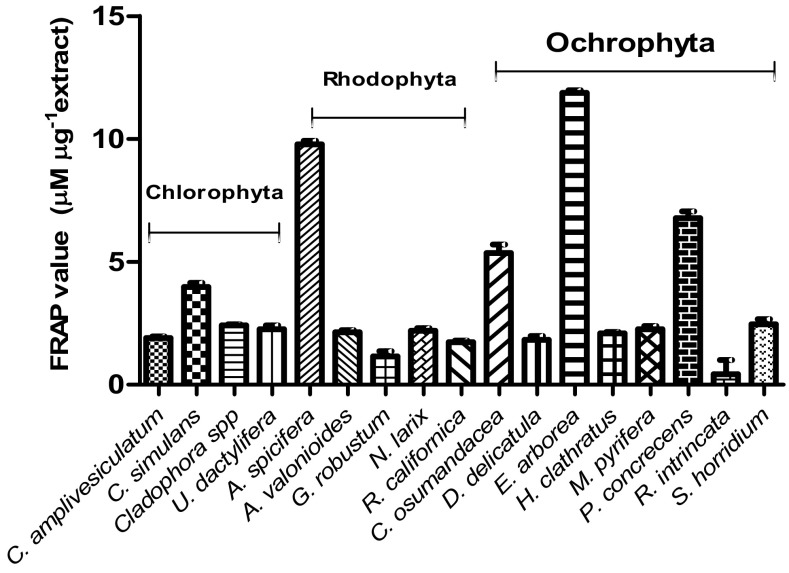
Antioxidant ability of macroalgal extracts for reducing Fe(III) by the FRAP values reflecting the ranged from 0.423 ± 0.58 µM FeSO4 µg−1 for Rosenvigea intricata to 11.8 ± 0.11 µM FeSO4 µg−1 for Eisenia arborea (Fig. 3). FRAP assays varied significantly among species [(F (16, 34) = 637.5, p = 0.0001]. The brown macroalga E. arborea showed significantly higher mean FRAP (11.9 µM FeSO4 µg−1), followed by red A. spicifera (9.8 µM FeSO4 µg−1), brown P. concrecens (6.8 µM FeSO4 µg−1), and brown C. osmundacea (5.4 µM FeSO4 µg−1). Brown macroalgae as a group had the highest FRAP value, with a combined mean of 4.6 ± 3.5 µM FeSO4 µg−1followed by red macroalgae with a combined mean of 3.1 ± 3.5 µM FeSO4 µg−1. The green macroalgae had the lowest FRAP value mean (2.6 ± 0.83 µM FeSO4 µg−1). However, there was no statistical difference among the antioxidant activities of green, red, and brown macroalgae [(F (2, 48) = 2.42, p = 0.099].
Fig. 3.
Ferric-reducing antioxidant power (FRAP) of macroalgal ethanol extracts expressed as µM equivalents of FeSO4
Total phenol content (TPC)
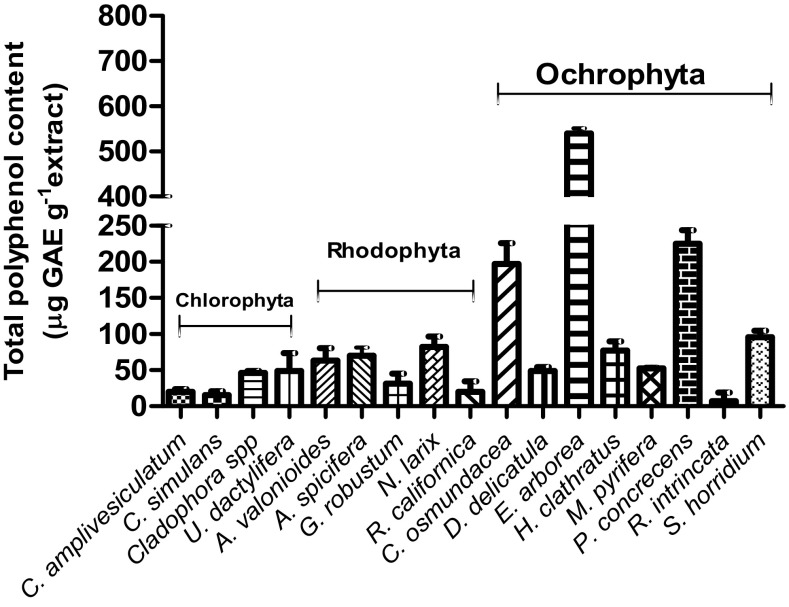
The total phenolic content varied among species from 7.16 ± 5.95 µg GAE g−1 for Rosenvigea intricata to 539.5 ± 11.88 µg GAE g−1 for E. arborea (Fig. 4). The phenolic content in E. arborea extract was significantly different, compared with the other species. The brown macroalgal group had the highest mean TPC (176.5 ± 174.4 µg GAE g−1), followed by red macroalgae (TPC = 45.6 ± 30.2 μg GAE g−1). The green macroalgal group had the lowest mean TPC (32.7 ± 17.82 μg GAE g−1). ANOVA and post hoc comparisons show that brown macroalgae were statistically higher than red and green macroalgae [(F (2, 64) = 8.26, p = 0.0001]. There was no statistical difference in mean TPC between the red and green macroalgae.
Fig. 4.
Total phenolic content of macroalgal ethanol extracts expressed as microequivalents of gallic acid (GAE)
Correlations between total phenolic content and antioxidant activity assays
A significant (p = 0.05) correlation between total TPC and different antioxidant scavenging assays of macroalgal extracts was shown by Pearson correlation analysis. For TPC versus DPPH scavenging activity: r = 0.416; TPC versus nitric oxide scavenging activity: r = 0.280; TPC versus FRAP activity: r = 0.620.
Discussion
Marine algae are a rich source of bioactive secondary metabolites, including phenols and polyphenols (Fernando et al. 2016). Antioxidant activity, an important property of bioactive algal compounds, has been ascribed to their reactive oxygen species scavenging ability, quenching singlet oxygen, reducing power, and chelating ability (Andrade et al. 2013; Maharana et al. 2015). The many antioxidant components in crude extracts of macroalgae make individual measurements different of each antioxidant. Therefore, several related assays are used to evaluate the antioxidant properties of macroalgae (Hafting et al. 2015).
Seventeen ethanol extracts were assayed as radical scavengers against DPPH and NO, and for reducing capacity by FRAP assay. Among the extracts, brown macroalgae had the highest antioxidant activity. Similar results have been observed by Wang et al. (2009), Kelman et al. (2012) and Mhadhebi et al. (2014). Eisenia arborea, Padina concrecens, and Cystoseira osmundacea, three of the brown macroalgae, possessed significantly greater antioxidant activity than the other macroalgae, indicating that they are potentially useful in manufacturing. Additionally, these species grow extensively along the coast of Baja California Peninsula due to its relative high reproductive capacity and abundance.
Antioxidant activity was equivalent to the commercial antioxidant BHT. Similarly, extracts of some species we tested, Brongniartella byssoides and Sargassum siliquastrum were similar to commercial antioxidants BHT and butylated hydroxyanisole (BHA) (Lim et al. 2002; Zubia et al. 2009). The high antioxidant activity of Baja California E. arborea is similar to the results of in Portugal (Raja et al. 2015). Cystoseira osmundacea in this study had high antioxidant activity determined by DPPH assay. DPPH scavenging of Cystoseira species extracts was reported by EC50 = 0.49, 0.58, 1.96, 4.94 mg mL−1 (Zubia et al. 2009; Andrade et al. 2013).
The FRAP assay is based on the ability of antioxidant components to reduce Ferric(III) to Ferrous(II) in a redox reaction that involves a single electron transfer. Phlorotannins in brown macroalgae are strong chelators of heavy metals, responsible for the chelating ability in several macroalgae: Turbinaria conoides (Devi et al. 2011), D ictyota dichotoma (Parthiban et al. 2013), Eisenia bicyclis (Machu et al. 2015), Cystoseira tamariscifolia (Custódio et al. 2016), among others (Maharana et al. 2015; Vizetto-Duarte et al. 2016). In our study, E. arborea, P. concrecens, and C. osmundacea had the higher values, similar to those reported by Kelman et al. (2012) for Turbinaria ornata (10.27 and 7.50 µM FeSO4 µg−1). In other studies, Sargassum extracts have high antioxidant activity (Yangthong et al. 2009; Lim et al. 2002); however, our assays gave very low results. Safari et al. (2015) attributed the disparity in antioxidant activity in studies to the season of collection, preparation of the extract, and other factors. In Mexico, Sargassum is particularly abundant along the coast of the Peninsula of Baja California, where 183,000 t per year is estimated to be harvested (Casas-Valdez et al. 2006). Therefore, its potential biological activities merit further research.
Nitric oxide plays a major role in promoting inflammatory response and toxicity is induced when reacting with oxide radicals to form peroxynitrite, which damages structural biomolecules and disrupts biological processes in animal (Soufli et al. 2016). Nitric oxide is generated when sodium nitroprusside reacts with oxygen to form nitrites. We found nine extracts that scavenged nitric oxide. Our results suggest that those macroalgal extracts could help in controlling inflammation, since NO is a potent mediator of physiological processes such as deregulated strong inflammatory responses (Soufli et al. 2016). Nitric oxide scavenging of some Cystoseira species and E. arborea show promise for treating inflammation (Mhadhebi et al. 2014; Lopes et al. 2012). Similar to our results, Codium tomentosum and Plocamium cartilagineum aqueous extracts and Fucus spiralis ethanol extract are potent nitric oxide scavengers (Valentão et al. 2010; Andrade et al. 2013).
Polyphenol compounds are natural antioxidants found in plants, and phlorotannins are polyphenol compounds formed by oligomers and polymers of phloroglucinol (1, 3, 5-trihydroxy benzene). As in our study, Yangthong et al. (2009) found significant differences in total phenolic content among the three macroalgal groups (brown, red, and green). Our results showed that polyphenol content was quite large in brown macroalgae compared with red and green macroalgae, as previously reported (Matanjun et al. 2008; Wang et al. 2009). Other studies found that polyphenols in brown macroalgae have extraordinary and effective antioxidant activities (Zubia et al. 2009; Machu et al. 2015), which suggest that higher total phenolic content can result in higher antioxidant capacity. The high phenolic content of Baja California E. arborea, P. concrecens, and C. osmundacea can explain their potent radical scavenging activity, since polyphenol compounds contain reducing properties, such as hydrogen or electron-donating agents, which contribute to free radical scavenger potential (Fernando et al. 2016). Although Acanthophora spicifera had low phenolic content, its extract had significant antioxidant activity. This is likely because this species belongs to the red algae family Rhodomelaceae that is rich in bromophenols, sharing one or several benzene rings, where the hydroxyl groups are substituted by a bromine group with potent antioxidant activities (Liu et al. 2011; Chakraborty et al. 2015). Other abundant compounds in A. spicifera are sulfated polysaccharides such as agaran, which possibly were co-extracted and might also support its antioxidant activity (Ganesan et al. 2008). Remarkably, reports show that there is a direct relationship between antioxidant activity, total phenolic content and concentration in herbs, vegetables, and macroalgae (Machu et al. 2015; Raja et al. 2015). We found a positive correlation between phenol content and scavenging capacity in several species, similar to extracts from Sargassum sp (Yangthong et al. 2009), Caulerpa sedoides (Mhadhebi et al. 2014), and E. arborea (Raja et al. 2015), as well as other reports (Osuna-Ruiz et al. 2016). Therefore, the results obtained from the present study suggested that the antioxidant activity might be due to the presence of polar phenolic compounds in the macroalgal extracts. Although previous data on the inhibition of cell proliferation with macroalgal extracts is very limited, previous result have shown no toxicity effects (Erfani et al. 2015; Kalaiselvan et al. 2016).
Conclusions
The antioxidant activities of 17 Baja California Peninsula macroalgal species clearly indicate that they possess antioxidant activity in varying degrees. To our knowledge, this is the first report on antioxidant activity of Rodhymenia californica, Rosenvigea intricata, and Cystoseira osmundacea extracts, as well as screening of antioxidant capacity and phenolic content of macroalgal species in the Baja California Peninsula. These phenolics compounds may play a role in the activities observed in ethanolic extracts. Since Eisenia arborea was found to have a potent antioxidant capacity and Macrocystis pyrifera are the most abundant macroalgae along this coast and exhibit high polyphenol content and antioxidant activity, the chemical characterization of their phlorotannins and other bioactive compounds merits further research, such as phlorotannins with cosmetical and medical applications.
Acknowledgements
The authors gratefully acknowledge the help of Dr. Rafael Riosmena-Rodríguez, Dr. Luz Elena Mateo Cid and Dr. Angela Catalina Mendoza-Gonzalez for the taxonomic algal identification. We also thanks the rechnical assistance was provided by M.Sc. Roberto Hernadez-Herrera and English editorial services by Ira Fogel of CIBNOR. This research was funded by CONACYT Mexico (INFR-2014-01/225924 and PDCPN2014-01/248033). P.A.T.R. is a recipient of a study fellowship (CONACYT).
Compliance with ethical standards
Conflict of interest
The authors have no affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Contributor Information
Ángel I. Campa-Cordova, Phone: +52 612-123-8484, Email: angcamp04@cibnor.mx
Carlos Angulo, Phone: +52 612-123-8484, Email: eangulo@cibnor.mx.
References
- Águila-Ramírez RN, Arenas-González A, Hernández-Guerrero CJ, González-Acosta B, Borges-Souza JM, Véron B, Pope J, Hellio C. Antimicrobial and antifouling activities achieved by extracts of seaweeds from Gulf of California, Mexico. Hidrobiológica. 2012;22(1):8–15. [Google Scholar]
- Andrade PB, Barbosa M, Matos RP, Lopes G, Vinholes J, Mouga T, Valentão P. Valuable compounds in macroalgae extracts. Food Chem. 2013;138(2):1819–1828. doi: 10.1016/j.foodchem.2012.11.081. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. Ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Casas-Valdez M, Hernandez-Contreras H, Marin-Alvarez A, Aguila-Ramirez RN, Hernandez-Guerrero CJ, Sanchez-Rodriguez I, Carrillo-Dominguez S. The seaweed Sargassum (Sargassaceae) as tropical alternative for goats’ feeding. Rev Biol Troph. 2006;54:83–92. doi: 10.15517/rbt.v54i1.14002. [DOI] [PubMed] [Google Scholar]
- Chakraborty K, Joseph D, Praveen NK. Antioxidant activities and phenolic contents of three red seaweeds (Division: Rhodophyta) harvested from the Gulf of Mannar of Peninsular India. J Food Sci Technol. 2015;52:1924–1935. doi: 10.1007/s13197-013-1189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custódio L, Silvestre L, Rocha MI, Rodrigues MJ, Vizetto-Duarte C, Pereira H, Barreira L, Varela J. Methanol extracts from Cystoseira tamariscifolia and Cystoseira nodicaulis are able to inhibit cholinesterases and protect a human dopaminergic cell line from hydrogen peroxide-induced cytotoxicity. Pharm Biol. 2016;54:1687–1696. doi: 10.3109/13880209.2015.1123278. [DOI] [PubMed] [Google Scholar]
- Devi GK, Manivannan K, Thirumaran G, Rajathi FA, Anantharaman P. In vitro antioxidant activities of selected seaweeds from Southeast coast of India. Asian Pac J Troph Med. 2011;4:205–211. doi: 10.1016/S1995-7645(11)60070-9. [DOI] [PubMed] [Google Scholar]
- Erfani N, Nazemosadat Z, Mahmoodrez M. Cytotoxic activity of ten algae from the Persian Gulf and Oman Sea on human breast cancer cell lines; MDA-MB-231, MCF-7, and T-47D. Pharmacogn Res. 2015;7:133–137. doi: 10.4103/0974-8490.150539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando SIP, Kim M, Kwang-Tae S, Jeong Y, You-Jin J. Antioxidant activity of marine algal polyphenolic compounds: a mechanistic approach. J Med Food. 2016;19(7):1–14. doi: 10.1089/jmf.2016.3706. [DOI] [PubMed] [Google Scholar]
- Ganesan P, Kumar CS, Bhaskar N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour Technol. 2008;99:2717–2723. doi: 10.1016/j.biortech.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Hafting JT, Craigie JS, Stengel DB, Loureiro RR, Buschmann AH, Yarish C, Edwards MD, Critchley AT. Prospects and challenges for industrial production of seaweed bioactives. J Phycol. 2015;51:821–837. doi: 10.1111/jpy.12326. [DOI] [PubMed] [Google Scholar]
- Hernández-Carmona G, Rodríguez-Montesinos Y, Casas-Valdez M, Vilchis M, Sánchez-Rodríguez M. Evaluation of the beds of Macrocystis pyrifera in the Baja California Peninsula, Mexico III. Summer 1986 and seasonal variation. Cienc Mar. 1991;17:121–145. [Google Scholar]
- Kalaiselvan I, Senthamarai M, Kasi PD. 2,3,7,8-TCDD-mediated toxicity in peripheral blood mononuclear cells is alleviated by the antioxidants present in Gelidiella acerosa: an in vitro study. Environ Sci Pollut Res Int. 2016;23:5111–5121. doi: 10.1007/s11356-014-3799-2. [DOI] [PubMed] [Google Scholar]
- Kelman D, Posner EK, McDermid KJ, Tabandera NK, Wright PR, Wright AD. Antioxidant activity of Hawaiian marine algae. Mar Drugs. 2012;10(2):403–416. doi: 10.3390/md10020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SN, Cheung PCK, Ooi VEC, Ang PO. Evaluation of antioxidative activity of extracts from a brown seaweed, Sargassum siliquastrum. J Agric Food Chem. 2002;50:3862–3866. doi: 10.1021/jf020096b. [DOI] [PubMed] [Google Scholar]
- Liu M, Hansen PE, Lin X. Bromophenols in marine algae and their bioactivities. Mar Drugs. 2011;9:1273–1292. doi: 10.3390/md9071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes G, Sousa C, Silva LR, Pinto E, Andrade PB, Bernardo J, Mouga T, Valentão P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE. 2012;7:e31145. doi: 10.1371/journal.pone.0031145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machu L, Misurcova L, Ambrozova J, Orsavova J, Mlcek J, Sochor J, Jurikova T. Phenolic content and antioxidant capacity in algal food products. Molecules. 2015;20:1118–1133. doi: 10.3390/molecules20011118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharana D, Das PB, Verlecar XN, Pise NM, Gauns M. Oxidative stress tolerance in intertidal red seaweed Hypnea musciformis (Wulfen) in relation to environmental components. Environ Sci Pollut Res Int. 2015;22:18741–18749. doi: 10.1007/s11356-015-4985-6. [DOI] [PubMed] [Google Scholar]
- Matanjun P, Mohamed S, Mustapha NM, Muhammad K, Ming CH. Antioxidant activities and phenolics content of eight species of seaweeds from North Borneo. J Appl Phycol. 2008;20:367–373. doi: 10.1007/s10811-007-9264-6. [DOI] [Google Scholar]
- Mhadhebi L, Mhadhebi A, Robert J, Bouraoui A. Antioxidant, anti-inflammatory and antiproliferative effects of aqueous extracts of three mediterranean brown seaweeds of the genus cystoseira. Iran J Pharm Res. 2014;13(1):207–220. [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Ochoa M, Murillo-Álvarez JI, Rodríguez Montesinos YE, Hernández Carmona G, Arvizu Higuera DL, Peralta Cruz J, Lizardi Mendoza J. Anticoagulant screening of marine algae from Mexico, and partial characterization of the active sulfated polysaccharide from Eisenia arborea. CICIMAR Oceán. 2009;24(1):41–51. [Google Scholar]
- Muñoz-Ochoa M, Murillo-Álvarez JI, Zermeño Cervantes LA, Martínez Díaz SF, Riosmena Rodríguez R. Screening of extracts of algae from Baja California Sur, Mexico as reversers of the antibiotic resistance of some pathogenic bacteria. Eur Rev Med Pharmacol Sci. 2010;14:739–747. [PubMed] [Google Scholar]
- Nagayama K, Iwamura Y, Shibata T, Hirayama I, Nakamura T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J Antimicrob Chemoth. 2002;50:889–893. doi: 10.1093/jac/dkf222. [DOI] [PubMed] [Google Scholar]
- Osuna-Ruiz I, López-Saiz CM, Burgos-Hernández A, Velázquez C, Nieves-Soto M, Hurtado-Oliva MA. Antioxidant, antimutagenic and antiproliferative activities in selected seaweed species from Sinaloa, Mexico. Pharm Biol. 2016;9:1–15. doi: 10.3109/13880209.2016.1150305. [DOI] [PubMed] [Google Scholar]
- Parthiban C, Saranya C, Girija K, Hemalatha A, Suresh M, Anantharaman P. Evaluation of in vitro antioxidant properties of some selected seaweeds from tuticorin coast. Int J Curr Microbiol App Sci. 2013;2:64–73. [Google Scholar]
- Raja R, Hemaiswarya S, Arunkumar K, Carvalho IS. Antioxidant activity and lipid profile of three seaweeds of Faro, Portugal. Braz J Bot. 2015;1:9–17. [Google Scholar]
- Safari P, Rezaei M, Shaviklo AR. The optimum conditions for the extraction of antioxidant compounds from the Persian Gulf green algae (Chaetomorpha sp.) using response surface methodology. J Food Sci Technol. 2015;52:2974–2981. doi: 10.1007/s13197-014-1355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Soufli I, Toumi R, Rafa H, Touil-Boukoffa C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther. 2016;7:353–360. doi: 10.4292/wjgpt.v7.i3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentão P, Trindade P, Gomes D, de Pinho PG, Mouga T, Andrade PB. Codium tomentosum and Plocamium cartilagineum: chemistry and antioxidant potential. Food Chem. 2010;119:1359–1368. doi: 10.1016/j.foodchem.2009.09.015. [DOI] [Google Scholar]
- Vizetto-Duarte C, Custódio L, Acosta G, Lago JH, Morais TR, Bruno de Sousa C, Gangadhar KN, Rodrigues MJ, Pereira H, Lima RT, Vasconcelos MH, Barreira L, Rauter AP, Albericio F, Varela J. Can macroalgae provide promising anti-tumoral compounds? A closer look at Cystoseira tamariscifolia as a source for antioxidant and anti-hepatocarcinoma compounds. Peer J. 2016;4:e1704. doi: 10.7717/peerj.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Jónsdóttir R, Ólafsdóttir G. Total phenolic compounds, radical scavenging and metal chelation of extracts form Icelandic seaweeds. Food Chem. 2009;11:6240–6248. [Google Scholar]
- Yangthong M, Hutadilok-Towatana N, Phromkunthong W. Antioxidant activities of four edible seaweeds from the southern coast of Thailand. Plant Foods Hum Nutr. 2009;64:218–223. doi: 10.1007/s11130-009-0127-y. [DOI] [PubMed] [Google Scholar]
- Zubia M, Fabre MS, Kerjean V, Lann KL, Pouvreau VS, Fauchon M, Deslandes E. Antioxidant and antitumoural activities of some phaeophyta from Brittany coasts. Food Chem. 2009;116:693–701. doi: 10.1016/j.foodchem.2009.03.025. [DOI] [Google Scholar]