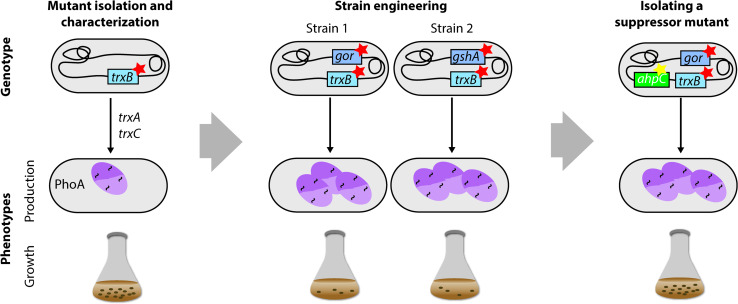
Fig. 8.
Combining evolutionary approaches and engineering to create E. coli strains enabling the efficient production of disulfide-containing proteins in the cytoplasm. A screening approach was used to isolate E. coli strains that allow the formation of disulfide bonds in the cytoplasm [150]. In the screen, PhoA, a periplasmic protein, which requires disulfide bonds for its activity, was produced without a signal sequence in a strain lacking chromosomal phoA. The activity of the signal-sequence-less PhoA served as an indicator for cytoplasmic disulfide bond formation. Subsequently, mutants with PhoA activity were screened for, which resulted in the isolation of trxB-deficient strains. Using an engineering approach, it was found that disulfide bond formation in the cytoplasm is even more efficient in trxB null mutants that are unable to either synthesize or reduce gluthathione (gshA − or gor −) [151]. However, these double mutants grow very poorly and require an exogenous reductant to achieve a reasonable growth rate. Finally, suppressor strains were isolated that grow well and still allow stable disulfide bond formation in the cytoplasm [152]