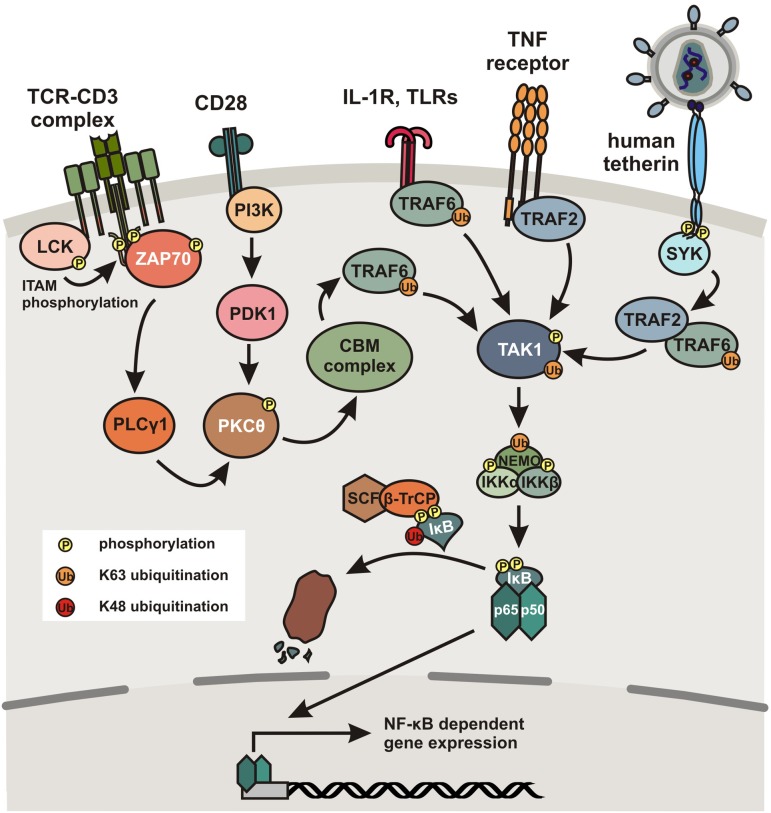
FIGURE 1.
Activation of the canonical NF-κB pathway. Simplified schematic presentation of NF-κB activation by different cellular receptors. Binding of the TCR-CD3 complex to antigens presented by MHC molecules induces LCK-dependent phosphorylation of ITAMs in the cytoplasmic tail of CD3. This in turn recruits ZAP70, which induces a signal cascade involving activation of PLCγ1 and PKC𝜃. Alternatively, PKC𝜃 can also be activated via a signal cascade including PI3K following binding of the TCR costimulatory factor CD28 to a B7 ligand. PKC𝜃 subsequently activates the CBM complex consisting of CARD11, BCL10 and MALT1, which activates TAK1 in a TRAF6 dependent manner. Ligand binding to TLRs or TNF receptors also induces TAK1 activation in a TRAF6- or TRAF2-dependent manner, respectively. In humans (and to a much lesser extent in chimpanzees), the cellular restriction factor tetherin also acquired the ability to activate NF-κB. Upon tethering of newly formed viral particles and clustering of tetherin dimers, the cytoplasmic tail of tetherin becomes phosphorylated at a conserved YxY motif by a Scr-family kinase in a RICH2-dependent manner, recruiting SYK, which initiates a signaling cascade involving TRAF2 and/or TRAF6 resulting in the activation of TAK1. TAK1 subsequently phosphorylates IKKβ at two serine residues in the activation loop to activate IKK. Furthermore, TRAFs activate NEMO by poly-ubiquitination, followed by activation of the catalytic subunits IKKα and IKKβ. Subsequently, IκB is phosphorylated and poly-ubiquitinated by the SCF/β-TrCP complex, which results in its proteasomal degradation, thereby releasing p50/p65 heterodimers, which translocate to the nucleus where they bind to specific κB binding sites and initiate NF-κB-dependent gene expression.