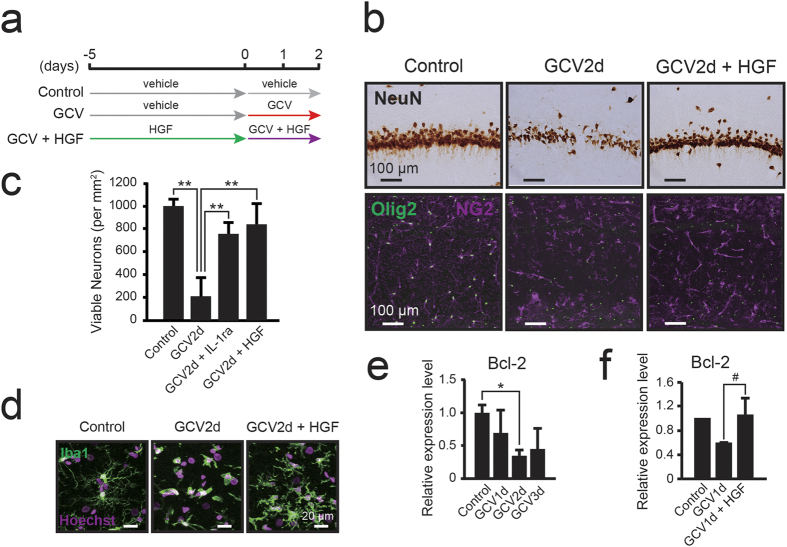
Figure 8. NG2 glial cell-derived HGF supports the survival of hippocampal neurons.
(a) Timeline of the experimental design for the treatment with mouse recombinant HGF (mrHGF). (b) Bright-field immunohistochemical observations of NeuN in the hippocampal CA1 region of NG2-HSVtk transgenic rats treated with vehicle (Control), GCV for 2 days (GCV2d), or co-administration of GCV and HGF (4.3 μg/day) for 2 days (GCV2d + HGF). (c) The number of viable neurons in NG2-HSVtk transgenic rats treated with vehicle (n = 2), GCV for 2 days (n = 3), co-administration of GCV and IL-1ra (GCV2d + IL-1ra, n = 3), or co-administration of GCV and HGF (GCV2d + HGF, n = 4). (d) Confocal images showing microglia (Iba1, green) in the hippocampal CA1 region of NG2-HSVtk transgenic rats treated with vehicle, GCV for 2 days, or co-administration of GCV and HGF for 2 days. Hoechst, cell nuclear staining. (e) Relative expression levels of Bcl-2 mRNA in the hippocampus of NG2-HSVtk transgenic rats treated with vehicle (Control, value set as 1.0) or GCV for 1, 2, or 3 days. n = 3 animals (Control, GCV3d) and 5 animals (GCV1d, GCV2d). (f) Relative expression levels of Bcl-2 mRNA in the hippocampi of rats treated with vehicle (Control, value set as 1.0, n = 2), GCV for 1 days (n = 3), or co-administration of GCV and HGF for 1 day (n = 3). Mean ± SD; *p < 0.05 or **p < 0.01, based on a one-way ANOVA followed by Tukey-Kramer test (c,e). #p = 0.05, based on a Student’s t-test analysis (f). Scale bars represent 100 μm (b) or 20 μm (d).