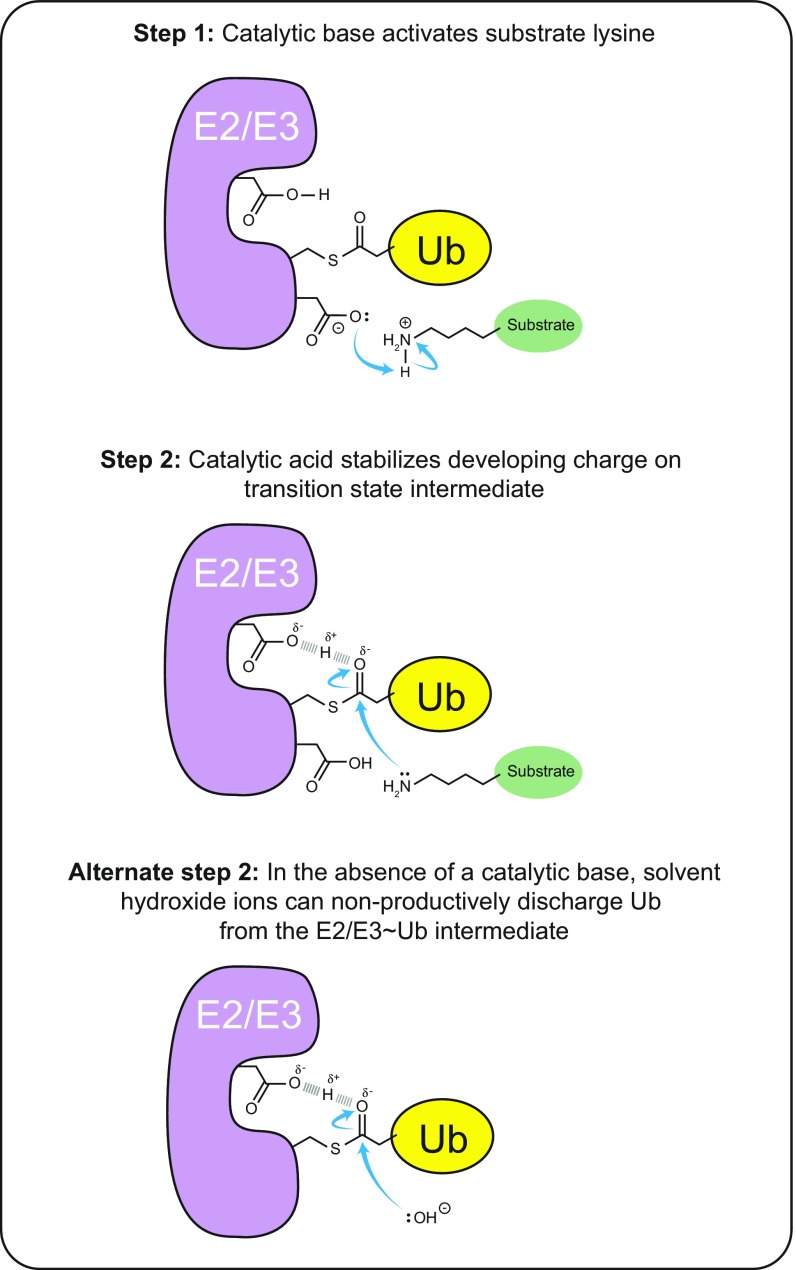
Fig. S2.
Summary of the generalizable roles of catalytic acid and catalytic base in aminolysis as inferred from representative E2 and E3 enzymes. Step 1: Lysine ubiquitination requires a catalytic base to activate its nucleophilic potential by deprotonating the positively charged nitrogen atom. Step 2: Subsequent nucleophilic attack by the activated lysine on the active site thioester-bound Ub requires stabilization of the developing negative charge on the carbonyl oxygen by a catalytic acid, which donates a proton to stabilize the transition state intermediate. Alternate step 2: Mutation of the catalytic base leads to accumulation of the Ub-charged state of the E2/E3 enzyme, which can be offset by nonproductive discharge of Ub to solvent by the nucleophilic attack of hydroxide ions. The choice of pathway is affected by the bulk pH of the solution and is viable because the intact catalytic acid stabilizes the transition state intermediate. Mutation of the catalytic acid leads to accumulation of a Ub-charged state of the E2/E3 enzyme, because neither an attacking lysine nor a hydroxide nucleophile can proceed through the transition state intermediate.