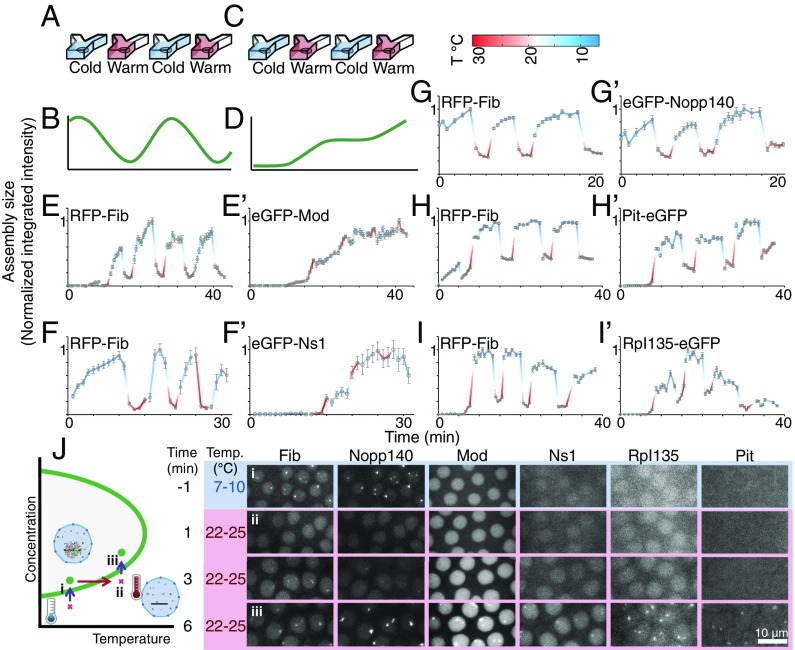
Fig. 3.
An in vivo assay to test the reversibility of assembly formation by nucleolar proteins. (A and C) A microfluidic device was used to switch between cold and warm temperatures in less than 1 min during NC14. (B and D) Schematics qualitatively illustrate predicted trends in assembly size for (B) a thermodynamic LLPS and (D) an active assembly. (B) A thermodynamic LLPS is expected to reversibly condense and dissolve by changing the temperature, whereas (D) an active assembly is expected to be slow at low temperatures, fast at high temperatures, and irreversible. (E–I and E′–I′) The integrated intensity at each temperature/time for high-concentration assemblies of different nucleolar proteins is determined, and mean SEM for n > 10 nuclei is normalized to its maximum level. Time 0 is when the experiment begins. The changes in the integrated intensity of the assemblies of (E′–I′) EGFP-tagged nucleolar proteins compared with those of (E–I) RFP-Fib are shown. (J) During early NC13, (i) Fib and Nopp140 assemblies are first to form at 8 °C to 10 °C. (ii) As soon as Fib assemblies appear, the embryos are shifted to 22 °C to 25 °C, in which the critical concentrations for Fib and Nopp140 are higher than the nucleoplasmic concentrations of these two proteins. This shift, therefore, causes the assemblies to dissolve. (iii) As the concentrations of Fib and Nopp140 increase by time, the assemblies reappear. The schematic in Left qualitatively illustrates the state of each step (i–iii) in the phase diagram. Images are maximum-projected. Time 0 marks the time when the temperature shift was applied.