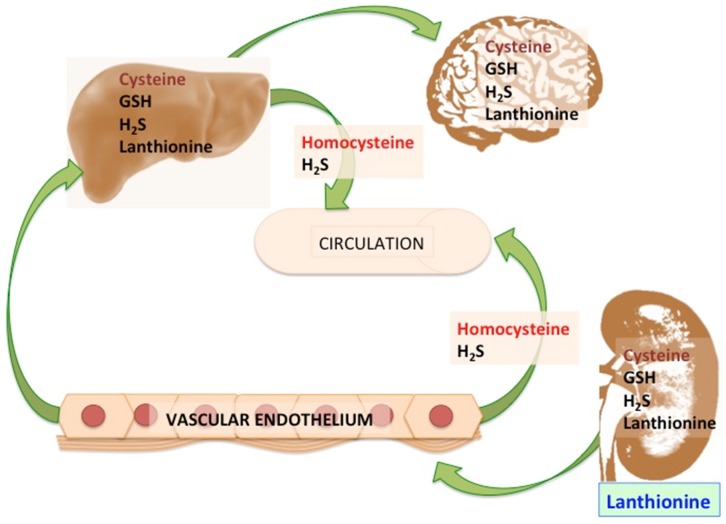
Figure 4.
A tissue metabolic crosstalk hypothesis. CBS and CSE are reported to be differentially expressed in organs and tissues, thus allowing transsulfuration to be carried out preferentially, but not exclusively, in the liver, while H2S biosynthesis may be performed by a number of other cell types. In this respect, it has been shown that, in the liver, CBS protein levels are significantly lower than those of CSE, while both these enzymes are more abundant in the liver than at the kidney level [37]. So the hypothesis of a metabolic crosstalk depends on the reported non-ubiquitous expression of either CBS or CSE in various organs, leading to the suggestion that the transsulfuration pathway is incomplete in some tissues, where only CBS or CSE are prevalently expressed. Cysteine, the main product of transsulfuration may be mainly produced where transsulfuration is fully active, while its utilization occurs at many other levels even where this pathway cannot take place (e.g., RBC). Cysteine is a building block for glutathione biosynthesis, or it is the major substrate for H2S, carried out by both CBS and CSE independently, even in the tissues where only CSE is significantly expressed, yielding lanthionine as the main product. Evidence supports that lanthionine is a retention product during chronic renal failure, despite low levels of H2S being detected in these patients [10]. On the other hand, homocysteine, an obligate metabolic intermediate in the conversion of methionine into cysteine and a major demethylated product of transmethylation reactions, can be further metabolized by both transsulfuration and H2S biosynthetic reactions. One of the main side products of the latter reaction, which can be carried out by both CBS and CSE independently, is homolanthionine (see also Figure 1 and Figure 2). It should be remarked, however, that, in contrast with previous observations, the transsulfuration pathway has been detected using brain cells and slices, thus demonstrating its contribution to the glutathione-dependent redox-buffering capacity [38]. It has also been shown that the endothelium may contribute, as the liver, to the homocysteine metabolic process in which the transsulfuration enzymes, CBS and CSE, are secreted by microvascular endothelial cells and hepatocytes, circulate as members of the plasma proteome, and may actively produce hydrogen sulfide from homocysteine in blood [39]. The latter pathway is possibly impaired in uremia due to high lanthionine circulating levels. The illustrated scheme may become more complex as new evidence is added which allows us to clarify the quantitative contribution of each tissue/organ to the disposal of homocysteine and the formation of cysteine or H2S by trassulfuration enzymes. Black letters refer to products, red letters refer to substrates; lanthionine is susceptible to quantitative urinary excretion (blue); cysteine is both a transsulfuration product and a substrate for H2S biosynthesis (purple red).