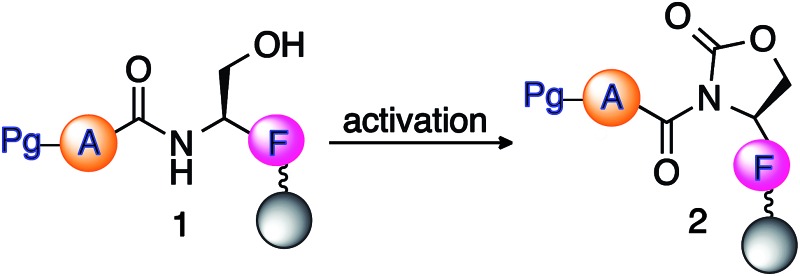
Table 2. Optimization of reaction conditions for the formation of the cyclic urethane moiety on solid support a .
| |||||
| Entry | Pg | DSC (equiv.) | Solvent | Time (h) | Conv. b (%) |
| 1 | 1b Fmoc | 10 | DCM | 17 | 40 |
| 2 | 1b Fmoc | 10 | DCM : DMF | 17 | 60 |
| 3 | 1b Fmoc | 10 | DMF | 17 | >99 |
| 4 | 1b Fmoc | 5 | DMF | 17 | 70 |
| 5 | 1b Fmoc | 10 | DMF | 3 | 30 |
| 6 | 1b Fmoc | 10 | DMF | 7 | 90 |
| 7 | 1c Ac | 10 | DMF | 17 | >99 |
| 8 | 1d tosyl | 10 | DMF | 17 | >99 |
| 9 | 1e Boc | 10 | DMF | 17 | >99 |
aReaction conditions: peptide 1 (25 mg, 0.87 mm g–1) on solid support was reacted with DSC (5–10 equiv.), DIEA (5–10 equiv.), and a crystal of DMAP in different solvents (3 mL) at room temperature for 3–17 h.
bConversion to cyclic urethane moiety 2 was calculated from the absorbance at 220 nm using HPLC.
