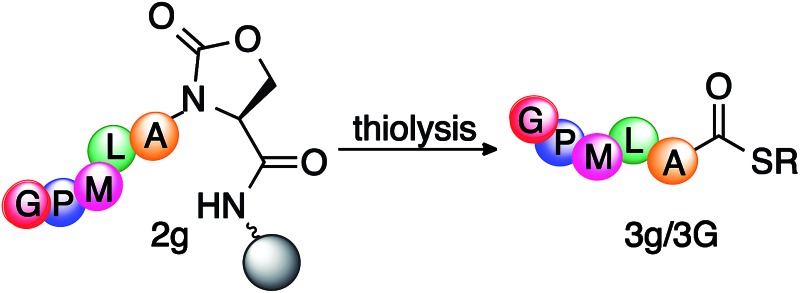
Table 3. Synthesis of peptide thioester 3g/3G from activated peptide Ac-GPMLA-Oxd-Rink AM 2g on solid support a .
| ||||||
| Entry | Base (equiv.) | Solvent | Temp. (°C) | Time (h) | Conv.
b
(%) |
|
| 3g | 3G | |||||
| 1 | — | DMF | RT | 20 | 10 | 15 |
| 2 | DBU (5 equiv.) | DMF | RT | 20 | 40 c | 60 |
| 3 | DIEA (20 equiv.) | DMF | RT | 20 | 60 c | 65 |
| 4 | PhSNa (0.5 equiv.) | DMF | RT | 20 | 85 c | 99 |
| 5 | PhSNa (0.5 equiv.) | ACN | RT | 20 | 15 | 25 |
| 6 | PhSNa (0.5 equiv.) | DMF | RT | 5 | 30 | 35 |
| 7 | PhSNa (0.5 equiv.) | DMF | 60 °C | 20 | 10 c | 70 c |
| 8 | PhSNa (0.5 equiv.) | DMF | 60 °C | 5 | 30 c | 99 |
| 9 | PhSNa (0.5 equiv.) | DMF | 60 °C | 3 | 50 c | 99 |
aReaction conditions: cyclic urethane-activated peptide Ac-GPMLA-Oxd 2g (25 mg, 0.7 mm g–1) on solid support was reacted with thiol (100 μL) and base (0.5–20 equiv.) in DMF (1 mL).
bConversion to peptide thioester Ac-GPMLA-COSR 3g or 3G was calculated from the absorbance at 220 nm using HPLC.
cActivated peptide was completely released from the resin but hydrolysis product was observed along with the thioester 3g/3G. SR = S-(CH2)2-OH or S-(CH2)2-COOC2H5, RT = room temperature, entries in bold: optimized conditions at room temperature. Thioesterification at high temperature leads to 5% epimerization.
