Table 1. Optimization of the dehydrogenative borylation reaction a .
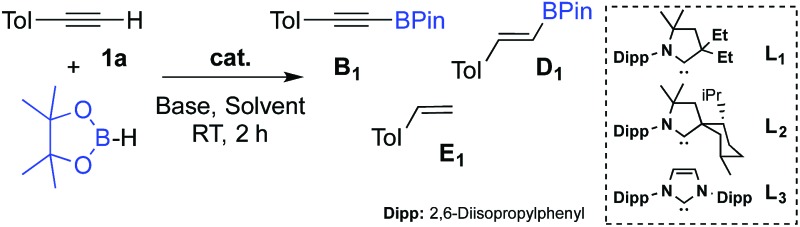
| ||||||||
| Entry | Cat. (mol%) | Base (mol%) | Solvent | Conc. (M) | 1a b (%) | B1 b (%) | D1 b (%) | E1 b (%) |
| 1 | — | — | C6D6 | 1.4 | 100 | 0 | 0 | 0 |
| 2 | L1CuOTf (1) | — | C6D6 | 1.4 | 86 | 0 | 6 | 6 |
| 3 | L1CuOTf (1) | Et3N (1) | C6D6 | 1.4 | 14 | 48 | 11 | 7 |
| 4 | CuOTf | Et3N (1) | C6D6 | 1.4 | 100 | 0 | 0 | 0 |
| 5 | L1CuOTf (1) | Et3N (2) | C6D6 | 1.4 | 1 | 70 | 14 | 12 |
| 6 | L1CuOTf (1) | Et3N (2) | CD2Cl2 | 1.4 | 12 | 42 | 6 | 26 |
| 7 | L1CuOTf (1) | Et3N (2) | THF-d8 | 1.4 | 5 | 64 | 12 | 17 |
| 8 | L1CuOTf (1) | Et3N (2) | CD3CN | 1.4 | 18 | 38 | 0 | 6 |
| 9 | L1CuOTf (1) | iPrNH2 (2) | C6D6 | 1.4 | 67 | 5 | 12 | 10 |
| 10 | L1CuOTf (1) | iPr2NH (2) | C6D6 | 1.4 | 47 | 11 | 13 | 7 |
| 11 | L1CuOTf (1) | iPr2NEt (2) | C6D6 | 1.4 | 10 | 28 | 45 | 3 |
| 12 | L1CuOTf (1) | BnNEt2 (2) | C6D6 | 1.4 | 14 | 53 | 8 | 7 |
| 13 | L1CuOTf (1) | DABCO (2) | C6D6 | 1.4 | 18 | 60 | 1 | 7 |
| 14 | L1CuOTf (0.25) | Et3N (0.5) | C6D6 | 1.4 | 37 | 36 | 15 | 6 |
| 15 | L1CuOTf (0.5) | Et3N (1) | C6D6 | 1.4 | 20 | 54 | 15 | 9 |
| 16 | L1CuOTf (2.5) | Et3N (5) | C6D6 | 1.4 | 4 | 83 | 4 | 7 |
| 17 | L 1 CuOTf (2.5) | Et 3 N (5) | C 6 D 6 | 0.1 | 1 | 98 | 0 | 1 |
| 18 | L2CuOTf (2.5) | Et3N (5) | C6D6 | 0.1 | 0 | 96 | 0 | 4 |
| 19 | L3CuOTf (2.5) | Et3N (5) | C6D6 | 0.1 | 0 | 92 | 0 | 8 |
aReactions were carried out in a test tube for 2 h at RT under an argon atmosphere using a 1 : 1 mixture (0.69 mmol) of p-tolylacetylene and pinacolborane.
bMeasured by NMR using 1,4-dioxane as an internal standard.
