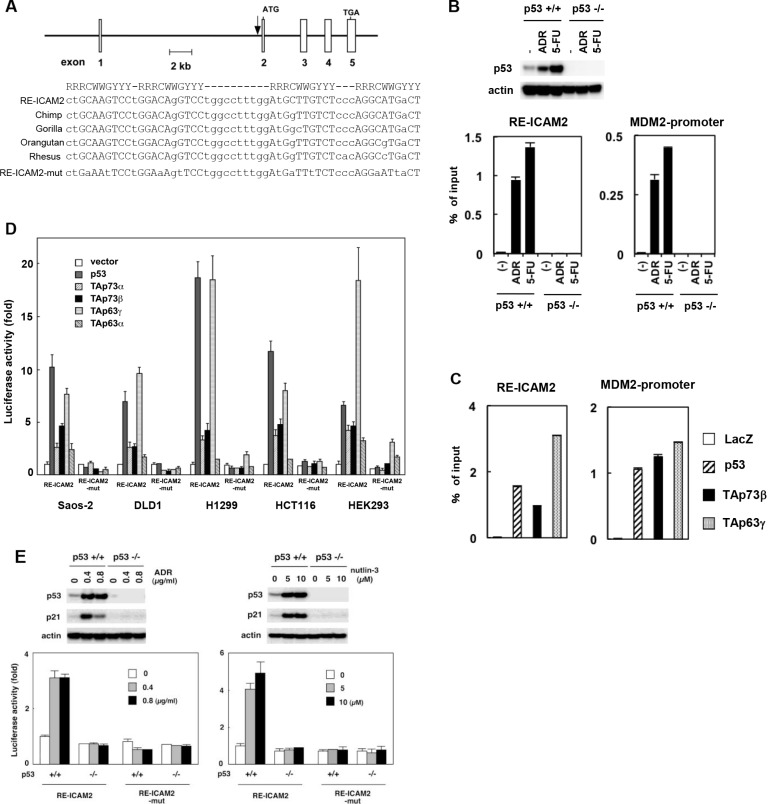
Figure 2. Regulation of ICAM2 transcription by p53 family.
(A) The genomic position and sequence of the p53 response element within the ICAM2 gene (RE-ICAM2). RE-ICAM2 is located within the first intron of the human ICAM2 gene and consists of four copies of the 10-bp p53 consensus-binding motif. The alignment of the conserved binding sites in chimpanzee, gorilla, orangutan, and rhesus sequences from the ICAM2 gene are shown. A mutated sequence corresponding to potentially critical nucleotides of RE-ICAM2 used in the luciferase assay is indicated in the bottom line (RE-ICAM2-mut). R represents purine; Y, pyrimidine; W, adenine or thymine. (B) Endogenous p53 binds to the RE-ICAM2 site in vivo. HCT116-p53(+/+) and HCT116-p53(−/−) cells were treated with 0.5 μg/mL ADR or 20 μg/mL 5-FU for 24 h and subjected to immunoblot analysis with an anti-p53 Ab (upper panels). ChIP assay for the presence of endogenous p53 protein at the RE-ICAM2 and MDM2 promoter was performed. PCR amplifications were performed in triplicates for each precipitation with primers surrounding each site. The data were normalized to the signal from input DNA and the mean and standard deviation are indicated by the bars (lower panels). (C) p53 family proteins bind to the RE-ICAM2 site in vivo. Crosslinked chromatin was extracted from Saos-2 cells following infection with Ad-LacZ, Ad-p53, Ad-p73β, and Ad-p63γ, and the cell lysates were then immunoprecipitated with an anti-FLAG antibody. PCR amplifications were performed as described above. (D) The RE-ICAM2 sequence was responsive to p53 family members. Cells were transiently transfected with the pGL3-promoter vector containing RE-ICAM2 (pGL3-RE-ICAM2) or its mutant (pGL3-RE-ICAM2-mut) along with a transfection-control plasmid expressing Renilla luciferase, phRG-TK. Cells were co-transfected with a control vector or a vector that expresses p53 family members 24 h prior to performing the luciferase assay. Luciferase activity was measured using the dual-luciferase reporter assay system with the Renilla luciferase activity as an internal control. All of the experiments were performed in quadruplicates, and the mean and standard deviations are indicated by the bars. (E) HCT116-p53(+/+) and HCT116-p53(−/−) cells were co-transfected with the pGL3-RE-ICAM2 or pGL3-RE-ICAM2-mut together with phRG-TK. At 4 h after transfection, cells were treated with ADR or Nutlin-3 for 24 h and subjected to dual-luciferase assay. Experiments were done in quadruplicates with standard deviations indicated. Activity in the control HCT116-p53(+/+) cells was set to 1. The top panel shows western blot analysis of p53 and actin in cells examined from each luciferase assay.