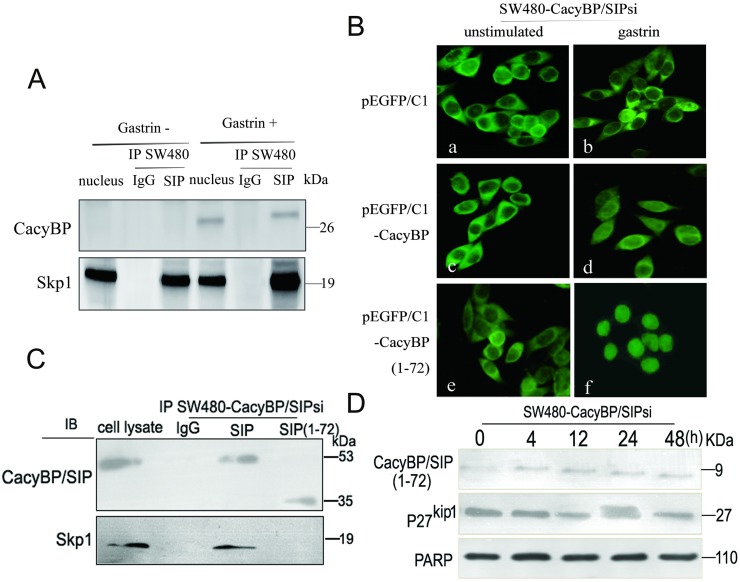
Fig 7. CacyBP/SIP interacts with the SCF component Skp1.
(A) Physical interaction between Skp1 and CacyBP/SIP in colon cancer cells. Nuclear lysates from HT29 and SW480 cells were immunoprecipitated with monoclonal antibodies against CacyBP/SIP or control IgG. The immunoprecipitates were then probed with an anti-Skp1 antibody using ECL-based detection. (B) HT29 and SW480 cells were transiently transfected with plasmids pEGFP/C1 producing a GFP-tagged version of either full-length CacyBP/SIP or the truncation mutant CacyBP/SIP (1–72) lacking the C-terminal 155 amino acids. Cells transfected with empty vector pEGFP/C1, served as a negative control. Transfectants were treated or not with gastrin (10−8 mol/L, 4 h), and EGFP fluorescence was analyzed under a confocal laser microscope. a,c and e unstimulated cells; b,d and f, cells after gastrin stimulation for 8 h. (C) Transfected cell lysates were subjected to immunoprecipitation using either anti-CacyBP/SIP antibody or IgG as control. Immune complexes were analyzed by immunoblotting using anti-Skp1 antibody with ECL-based detection. (D) After treatment with 10−8 mol/L gastrin for 8 h, lysates from the transfected cells were evaluated by Western blot analysis. Equal amounts of total cellular protein (40 μg) were subjected to SDS-PAGE followed by Western blot analysis for p27kip1. PARP was used as a nuclear protein loading standard.