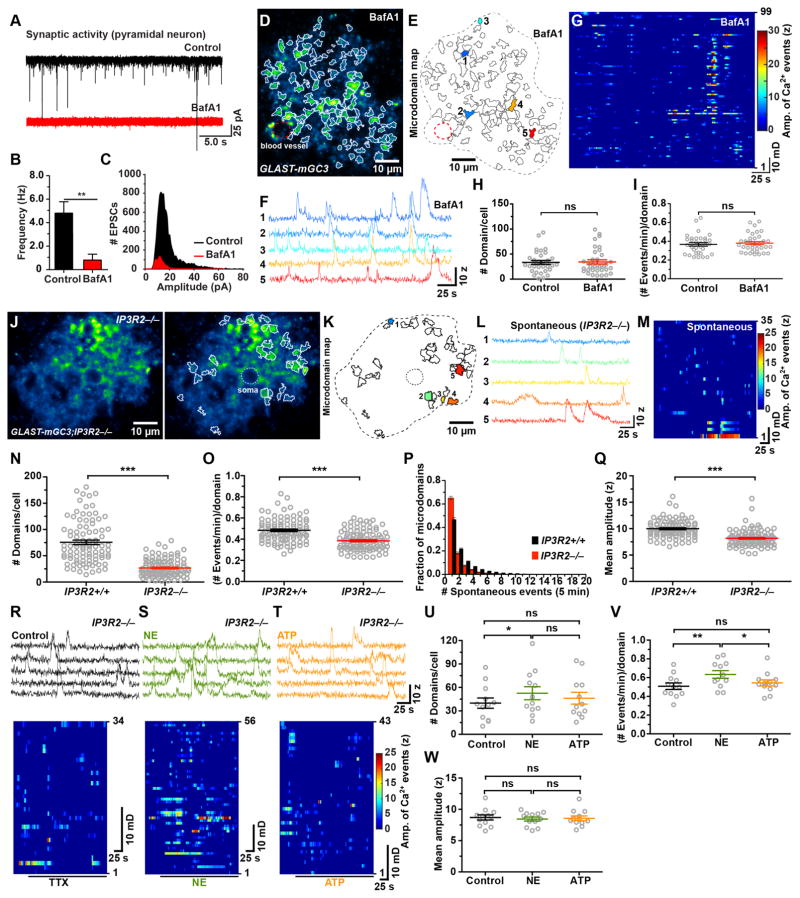
Figure 4. Spontaneous microdomain Ca2+ transients persist in the absence of neurotransmitter release and ER-dependent Ca2+ release (See also Figure S3 and Movie S3).
(A) Spontaneous EPSCs recorded from cortical pyramidal neurons in control conditions (black trace) and after treatment with veratidine (10 μM) and bafilomycin A1 (4 μM, BafA1, red trace).
(B) Histogram of the frequency of spontaneous EPSCs recorded in control conditions and after treatment with veratidine and BafA1. Data shown as mean ± SEM. N = 7 (untreated) N = 5 (BafA1) cells from control mice (GLAST-mGC3 or mGCaMP3/+). **p < 0.009, unpaired two-tailed Student’s t-test.
(C) Histogram of the amplitudes of spontaneous EPSCs recorded in control conditions and after treatment with veratidine and BafA1.
(D) Image of one astrocyte from a GLAST-mGC3 mouse showing median intensity projection (pseudocolored) from 540 frames (left) in an acute brain slice treated with veratridine and BafA1.
(E) Map of 99 spontaneously active microdomains recorded in veratridine and BafA1.
(F) Intensity versus time traces of 5 microdomains (corresponding to colors in E) showing characteristics of Ca2+ transients in veratridine and BafA1.
(G) Raster plot displaying Ca2+ transients from 99 microdomains in veratridine and BafA1.
(H, I) Graphs of number of microdomains per cell (H) and frequency of events per microdomain (I) recorded in control and in veratridine and BafA1. Data shown as mean ± SEM. N = 33 (untreated) and 36 (BafA1) cells from GLAST-mGC3 mice. ns: not significant, two-tailed Student’s t-test.
(J) Image of one astrocyte from a GLAST-mGC3;IP3R2−/−mouse showing median intensity projection (pseudocolored) from 260 s (left). Map of spontaneously active microdomains (in TTX (1μM)) overlaid on image (right).
(K) Map of all spontaneously active microdomains in 260 s (35 domains). Dashed line indicates cell border.
(L) Intensity versus time traces of five microdomains (corresponding to colors in B) showing characteristics of Ca2+ transients.
(M) Raster plot displaying Ca2+ transients from active regions in 260 s.
(N – Q) Graphs of number of microdomains per cell (N), frequency of events per domain (O), number of Ca2+ transients observed per microdomain (P) and mean amplitude (Q) in GLAST-mGC3 (Control, 94 cells) and GLAST-mGC3;IP3R2−/−(104 cells) mice. Data shown as mean ± SEM. *** p < 0.0001, unpaired two-tailed non-parametric Mann-Whitney test.
(R – T) Intensity versus time plots for 5 microdomains (top) and raster plots (bottom) displaying spontaneous Ca2+ transients (in control, black) and NE (10 μM, green) and ATP (100 μM, orange) from GLAST-mGC3;IP3R2−/−mice. All experiments were done in the presence of 1 μM TTX.
(U – W) Graphs of number of microdomains per cell (U), frequency of events per domain (V), and mean amplitude (W) of spontaneous Ca2+ transients (in TTX, black) and NE (10 μM, green) and ATP (100 μM, orange) from GLAST-mGC3;IP3R2−/− mice. Data shown as mean ± SEM. N = 12 cells for each condition. ns: not significant, p > 0.05, ** p < 0.01 and * p < 0.01 repeated measure one-way ANOVA analysis with Tukey’s multiple comparisons post hoc test.