Summary Statement
An association between perioperative hyperglycemia and adverse outcomes has been established in surgical patients, 1-3 with morbidity being reduced in those treated with insulin.5-6 A practical treatment algorithm and literature summary is provided for surgical patients with diabetes and hyperglycemia.
Keywords: Inpatient hyperglycemia, perioperative hyperglycemia, diabetes and surgery
Introduction
A substantial body of literature demonstrates a clear association between perioperative hyperglycemia and adverse clinical outcomes.1-3 The risk for post-operative complications and increased mortality relates to both long-term glycemic control and to the severity of hyperglycemia on admission and during the hospital stay.2 The underlying mechanism(s) relating hyperglycemia to poor outcomes is not completely understood. Past and current studies point to physiologic changes that occur in the hyperglycemic state that may contribute to poor outcomes. Elevated blood glucose levels impair neutrophil function, cause an overproduction of reactive oxygen species, free fatty acids and inflammatory mediators. These pathophysiologic changes contribute to direct cellular damage, vascular and immune dysfunction.4 Substantial evidence indicates that correction of hyperglycemia with insulin administration reduces hospital complications and decreases mortality in cardiac5 and general surgery patients.6 However, optimal glucose management during the perioperative period is widely debated. Recent randomized controlled trials targeting conventional targets for glycemic control do not demonstrate the significant risk of hypoglycemia7,8 as seen in prior studies using insulin to maintain tight blood glucose control.9 The pendulum of inpatient care has since moved toward more moderate and individualized glycemic targets.
This manuscript reports on the prevalence, diagnosis and pathophysiology of perioperative hyperglycemia and provides a practical outline for the management of surgical patients with diabetes and hyperglycemia.
Metabolic Consequences of Surgical Stress and Anesthesia
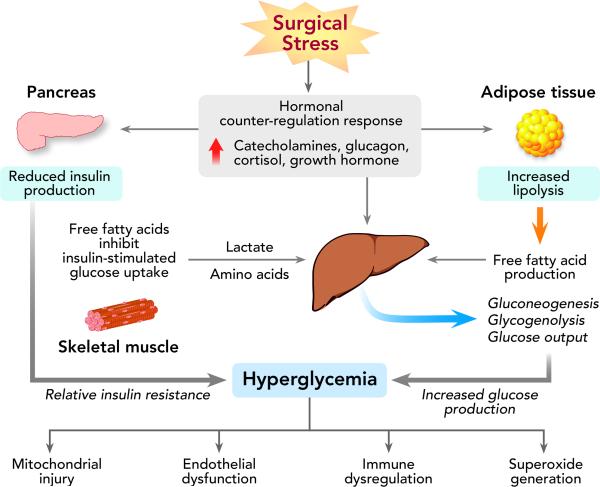
During the fasting state, normal subjects maintain plasma glucose levels between 60-100 mg/dl (3.3-5.5 mmol/l). The stress of surgery and anesthesia alters the finely regulated balance between hepatic glucose production and glucose utilization in peripheral tissues. An increase in the secretion of counterregulatory hormones (catecholamines, cortisol, glucagon, and growth hormone) occurs, causing excessive release of inflammatory cytokines including tumor necrosis factor-α, interleukin-6 and interleukin-1β (Figure 1).10 Cortisol increases hepatic glucose production, stimulates protein catabolism and promotes gluconeogenesis, resulting in elevated blood glucose levels.11 Surging catecholamines increase glucagon secretion and inhibit insulin release by pancreatic β-cells.4 Additionally, the increase in stress hormones leads to enhanced lipolysis and high free fatty acid (FFA) concentrations. Increased FFAs have been shown to inhibit insulin-stimulated glucose uptake12 and limit the intracellular signaling cascade in skeletal muscle responsible for glucose transport activity.13 Evidence also suggests that TNF-α interferes with the synthesis and/or translocation of the glucose transporter GLUT-4 reducing glucose uptake in peripheral tissues.14 These processes result in an altered state of insulin action, leading to a relative state of insulin resistance which is most pronounced on the first postoperative day and may persist for 9-21 days following surgery.15
Figure 1.
The Surgical Stress Response
Preoperative carbohydrate loading is becoming a more frequent surgical practice because it may counteract the state of insulin resistance that occurs due to stress and starvation. The Enhanced Recovery After Surgery (ERAS) program advocates carbohydrate-rich drinks up to two hours before surgery. This avoids the catabolic state associated with starvation, and has been demonstrated to increase insulin sensitivity16 and decrease the risk of postoperative hyperglycemia.17 Particularly in patients undergoing major abdominal surgery, carbohydrate loading has been associated with a reduced hospital length of stay.18
The magnitude of the counterregulatory response relates to the severity of surgery and the type of anesthesia.19 Anatomic location, invasiveness of the procedure, intraoperative fluids and nutritional support have all been linked to glucose elevation and the duration of stress hyperglycemia. Surgeries involving the thorax and abdomen are associated with a more pronounced and prolonged duration of hyperglycemia when compared to peripheral procedures.19 Laparoscopic procedures (versus open) demonstrate a decreased incidence of insulin resistance and hyperglycemia.15
Type of anesthesia also influences the hyperglycemic response during surgery. General anesthesia is more frequently associated with hyperglycemia and higher levels of catecholamines, cortisol and glucagon than local or epidural anesthesia.20 Volatile anesthetic agents inhibit insulin secretion21 and increase hepatic glucose production.22
Prevalence of Hyperglycemia and Diabetes in Surgical Patients
Perioperative hyperglycemia is reported in 20-40% of patients undergoing general surgery2,23,24 and approximately 80% of patients after cardiac surgery.5,25 A recent report examining point-of-care glucose testing in 3 million patients, across 575 American hospitals, reported a prevalence of hyperglycemia (BG >180 mg/dl, 10 mmol/l) as 32% in both intensive care (ICU) patients and non-ICU patients.26 Most patients with hyperglycemia have a known diagnosis of diabetes. However, 12-30% of patients who experience intra and/or post-operative hyperglycemia do not have a history of diabetes before surgery,2 a state often described as ‘stress hyperglycemia.’27 Stress hyperglycemia typically resolves as the acute illness or surgical stress abates. However, cross-sectional and longitudinal studies show that between 30-60% of these patients have impaired carbohydrate intolerance when assessed by oral glucose tolerance testing after hospital discharge.28 Furthermore, 60% of patients admitted with new hyperglycemia had confirmed diabetes at 1 year.28 Measurement of HbA1c in patients with hyperglycemia during hospitalization provides the opportunity to differentiate patients with stress hyperglycemia from those with diabetes who were previously undiagnosed.29 The Endocrine Society guidelines indicate that patients with hyperglycemia and HbA1C of 6.5% or higher can be identified as having diabetes.30
Preoperative period
Few studies have examined the effects of preoperative blood glucose levels on outcomes and there is a paucity of data for best preoperative glucose management. A retrospective analysis of 61,000 patients undergoing elective non-cardiac surgery demonstrated that one-year mortality was significantly related to preoperative blood glucose (BG). Crude incidence of mortality was 3-5% at one year in patients with preoperative BG between 60 and100 mg/dL (3.3-5.5 mmol/l) versus 12% in patients with BG > 216 mg/dL (12 mmol/l).31 Han et al32 reported that a preoperative hemoglobin A1c (HbA1c) level > 8% was an independent risk factor for wound complications (odds ratio [OR] 6.07; 95% confidence interval [CI], 1.12 to 33.0) in patients with type 2 diabetes undergoing total knee arthroplasty. Similarly, Dronge et al33 reported that among 490 diabetic patients who underwent major non-cardiac surgery, HbA1c level >7% was significantly associated with increased infectious complications with an adjusted odds ratio of 2.13 (95% confidence interval, 1.23-3.70) compared to patients with HbA1c < 7%. The relationship between peripheral BG and cerebral glucose is (as measured by intracerebral microdialysis catheters) is complicated in injured brain tissue and uncoupling can occur in the linear relationship. However, a review of the data does suggest that perioperative hyperglycemia in neurosurgical patients is a marker of poor outcomes.74 A recent study of 918 craniotomy or neurosurgical procedures for spine cases demonstrated increasing number of post-operative complications with increasing blood glucose.75 Halkos et al34 reported that preoperative HbA1c levels >7% in patients undergoing primary, elective coronary artery bypass surgery (CABG) had a higher unadjusted 5-year mortality compared with patients with HbA1c < 7%. These studies indicate that poor preoperative glycemic control is associated with an increased rate of complications and reduced long-term survival after surgery. Optimizing preoperative glucose management may improve outcomes; however, no prospective randomized studies have determined the importance of pre-operative control and clinical outcome.
Diabetes, Fasting and Feeding
The Joint Commission recommends a nutritional assessment occur within 24 hours of admission for all surgical patients. This is an important component of the preoperative evaluation. Most patients with and without diabetes tolerate fasting for several hours up to a few days without increased risk of malnutrition or perioperative complications. Nutritional support, with dextrose containing solutions is not indicated in diabetic patients fasting for periods less than 24-48 hours.
Prolonged fasting is avoided in patients with diabetes. Low carbohydrate diets facilitate insulin dosing and result in improved glucose control.35 The metabolic needs for most hospitalized patients can be supported by providing 25-35 calories/kg/day. Critically ill patients require decreased caloric intake approximating 15-25 calories/kg/day.35 A diet between 1800-2000 calories/day is appropriate for most patients.35 A meta-analysis examining diabetic enteric feeding formulas demonstrated that low-carbohydrate high–monounsaturated fatty acid are preferable to standard high-carbohydrate formulas in hospitalized patients with type 1 and type 2 diabetes.36 The postprandial rise in blood glucose was reduced by 18-29 mg/dL with these formulations, demonstrating improved glucose control in diabetics.36
Intraoperative period
Most investigations into the effects of intraoperative glucose control and outcomes have focused on the cardiac surgery population. In a retrospective study of 409 patients undergoing cardiac surgery, Gandhi et al37 reported that for each incremental change in intraoperative BG by 20 mg/dL (1.1mmol/l) above 100 mg/dL (5.5 mmol/l), there was a 30% increase in occurrence of adverse events including pulmonary and renal complications and death. Another study correlated glucose levels with risk of infection, atrial fibrillation, heart failure, myocardial infarction, pericarditis, neurologic complications, and pulmonary complications.38 Thirteen percent of patients with BG levels < 200 mg/dL (11.1 mmol/l) experience complications, versus 36% with glucose > 200 mg/dL (11.1 mmol/l) and 63% of patients with glucose ≥ 250 (13.9 mmol/l) mg/dL. In contrast to these observational studies, a randomized controlled trial of 400 diabetic and non-diabetic surgery patients assigned to receive continuous insulin infusion to maintain intraoperative glucose level between 80 and 100 mg/dL (4.4-5.6 mmol/l), versus those treated only for glucose levels > 200 mg/dL (11.1 mmol/l), reported more deaths and strokes in the intensive treatment group.39 A meta-analysis of 5 randomized controlled trials in 706 cardiac surgery patients reported that rigorous intraoperative glycemic control decreased the infection rate compared to conventional therapy but did not decrease mortality.40
Postoperative period
Studies in cardiac, general surgery and ICU patients have shown a clear association between inpatient hyperglycemia (>180 mg/dL, 10 mmol/l) and adverse clinical outcomes including surgical site infections, delayed wound healing and increased length of stay.1-3,24 Treating elevated BG has been reported to decrease morbidity.5,9 Numerous well-designed multicenter trials have shown that intensive insulin treatment results in a higher incidence of hypoglycemia and increased mortality compared to moderate glucose control.7,41,42
Several randomized controlled trials (all in cardiac surgery) have attempted to evaluate the ideal blood glucose target in the post-operative period to optimize outcomes and minimize harm.25,43-45 Desai et al43 randomized 189 patients to intensive insulin therapy (target 90 to 120 mg/dL, 5-6.7 mmol/l) or to conventional control (target 121 to 180 mg/dL, 6.7-10 mmol/l) testing the hypothesis that a liberal blood glucose target is non-inferior to intensive control for outcomes following first-time CABG surgery. There were no differences in deep sternal wound infection, pneumonia, perioperative renal failure, or mortality. However, the strict glucose control group took longer to reach target range, had a greater number of measurements outside of the target range, and more patients with hypoglycemic events. Pezzella et al44 assessed the long-term mortality in diabetics and non-diabetics randomized to intensive versus conventional control. There was no difference in health-related quality of life or survival between groups over a 40-month follow-up period demonstrating that long-term outcomes were not worse when blood glucose was maintained in liberal target range. Lazar et al45 investigated the effects of a target glucose of 90-120 mg/dL (5-6.7 mmol/l) versus 120-180 mg/dL (6.7-10 mmol/l) on clinical outcomes in 82 diabetic patients undergoing CABG. The study reported no differences in perioperative complications, hospital length of stay and mortality between the groups. The GLUCO-CABG trial25 randomized both diabetic and non-diabetic patients to intensive control (100-140 mg/dL, 5.5-7.8 mmol/l) or conventional glucose control (141-180 mg/dL, 7.8-10 mmol/l) following coronary bypass surgery. A significant difference was not detected between groups when evaluating a composite of complications including mortality, wound infection, pneumonia, bacteremia, respiratory failure, acute kidney injury, and major cardiovascular events. However, we observed heterogeneity in treatment effect according to diabetes status, with no differences in complications among patients with diabetes, but lower rates of complications in subjects without diabetes treated with intensive compared with conservative regimen. In agreement with these findings, a recent study by Blaha et al46 in 2,383 cardiac surgery patients treated to a target glucose range between 80 to 110 mg/dL (4.4-6.1 mmol/l) reported a reduction in postoperative complications only in non-diabetic patients (21% vs. 33%, relative risk 0.63, CI 0.54-0.74) without significant benefit of intensive therapy in patients with diabetes. These results indicate that at this time, institutions should target post-operative blood glucose levels between 140 and 180 mg/dL for cardiac surgery patients.
In general surgery patients, observational and prospective randomized studies have shown that hyperglycemia is associated with increased rates of morbidity and mortality.1-3,6,24 Improved glycemic control reduces the rate of hospital complications.3,6 In a cross-sectional study, patients with glucose levels between 110 and 200 mg/dL (6.1 mmol/l -11.1 mmol/l) versus patients with glucose levels >200 mg/dL (11.1 mmol/l) had respectively, a 1.7-fold and 2.1-fold increased mortality compared to those with glucose levels <110 mg/dL (6.1 mmol/l).47 The risk of postoperative complications in general surgery patients relates to the severity of hyperglycemia, with a higher risk observed in patients without a history of diabetes (stress-induced hyperglycemia) compared to those with a known diagnosis of diabetes.1-3
Glycemic Targets
Glycemic targets recommended by several organizations are shown in Table 1. The Society for Ambulatory Anesthesia (SAMBA) recommends intraoperative blood glucose levels <180 mg/dL (10 mmol/l).48 The American Association of Clinical Endocrinologists (AACE) Task Force and the American Diabetes Association (ADA) recommend target glucose levels between 140 and 180 mg/dL (7.7-10 mmol/l) in critically ill patients.49 The Society of Critical Care Medicine (SCCM) advises treatment be triggered at blood glucose levels ≥ 150 mg/dl (8.3 mmol/l0 with a goal to maintain blood glucose below that level, and absolutely <180 mg/dL (10 mmol/l).50 The Society of Thoracic Surgeons (STS) Practice Guidelines recommend maintaining serum glucose levels ≤ 180 mg/dL (10 mmol/l) for at least 24 hours after cardiac surgery.51 The American College of Physicians (ACP) advocates against intensive insulin therapy in patients with or without diabetes in surgical and medical ICUs. The ACP target blood glucose range is 140-200 mg/dL (7.7-11.1 mmol/L) for patients with or without diabetes.52
Table 1.
Society Guidelines Recommendations for Treatment of Perioperative Hyperglycemia and Diabetes
| Ambulatory Surgery | ICU | Non-ICU | |
|---|---|---|---|
| SAMBA 48 | SC rapid-acting insulin analogs are preferred over IV or SC regular insulin Treatment goal: Intraoperative blood glucose levels <180 mg/dL (10 mmol/L) |
||
| ADA/AACE 49 | Initiate insulin therapy for glucose >180 mg/dL (10 mmol/L). Treatment goal: For most patients, target a glucose level between 140–180 mg/dL (7.7-10 mmol/L). Glucose target between 110–140 mg/dL (6.1-7.7mmol/L) may be appropriate for select patients, if achievable without significant risk for hypoglycemia. |
Treatment goal: If treated with insulin, pre-meal glucose targets should generally be <140 mg/dL (<7.7 mmol/L), with random glucose levels <180 mg/dL (10 mmol/L). | |
| ACP 52 | Recommends against intensive insulin therapy in patients with or without diabetes in surgical/medical ICUs. Treatment goal: Target glucose is between 140-200 mg/dL (7.7-11.1mmol/L) in patients with or without diabetes. |
||
| Critical Care Society 50 | BG >150 mg/dL (8.3 mmol/L) should trigger insulin therapy. Treatment goal: Maintain glucose <150 mg/dL (8.3 mmol/L) for most patients in ICU. |
||
| Endocrine Society 30 |
Treatment goal: Target premeal blood glucose <140 mg/dL (7.7 mmol/L) and random glucose <180 mg/dL (10 mmol/L). Higher target glucose <200 mg/dL (11.1 mmol/l) is acceptable in patients with terminal illness and/or with limited life expectancy or at high risk for hypoglycemia. |
||
| Society of Thoracic Surgeons 51 | Continuous insulin infusion preferred over SC or intermittent IV boluses. Treatment goal: Recommend glucose <180 mg/dL (10 mmol/L) during surgery, ≤110 mg/dL (6.1 mmol/L) in fasting and pre-meal states. |
||
| Joint British Diabetes Societies 53 | Initiate insulin therapy for glucose >10 mmol/L (180 mg/dL). Target blood glucose levels in most patients are between 6 and 10 mmol/L (108–180 mg/dL) with an acceptable range of between 4 and 12 mmol/L (72–216 mg/dL). |
SAMBA: Society for Ambulatory Anesthesia; AACE/ADA: American Association of Endocrinologists and American Diabetes Association joint guidelines; ACP: American College of Physicians; ADA: American Diabetes Association; ICU: intensive care unit; IV: intravenous; SC: subcutaneous.
For patients in non-ICU settings, the Endocrine Society and the ADA/AACE Practice Guidelines recommended a target pre-meal glucose of <140 mg/dL (7.7 mmol/l) and a random BG of <180 mg/dL (10 mmol/l) for patients treated with insulin.30,49 The Joint British Diabetes Societies guideline, for the perioperative management of adult patients, recommends to start insulin treatment when glucose levels are >10 mmol / l (180 mg/dL). The Societies target a glucose range between 6 -10 mmol/l (108–180 mg/dL) but with acceptable values 4-12 mmol/l (72–216 mg/dL).53
Pre-Operative Glycemic Management
Treatment recommendations for type 2 diabetics using home medications are based on numerous factors. Type of diabetes, nature and extent of the surgical procedure, length of pre and post-operative fasting, type and frequency of daily medication, and state of metabolic control preceding surgery are taken into consideration when determining pre-operative medication use and dose.
There is a lack of randomized controlled trials demonstrating the role of oral medication before surgery. Generally, based on pharmacology and small studies, the use of most oral antidiabetic agents is recommended up to the day before surgery. Certain medications may be safely continued on the day of surgery (Table 2). It has been recommended that sulfonylurea and insulin secretogogues be discontinued the day of surgery as means to limit the risk of hypoglycemia.30,48 The SAMBA consensus statement on perioperative BG management in diabetic patients recommends that metformin may be taken the day before surgery, and restarted on the day of surgery when normal diet is resumed.48 However, the Joint British Diabetes Societies guideline allows metformin to be continued on the day of surgery for patients undergoing only a short starvation period (one missed meal).53 In patients undergoing procedures with use of intravenous contrast dye or with long expected surgical times, metformin is stopped when the preoperative fast begins, and restarted post-operatively with normal diet resumption. Should renal dysfunction (glomerular filtration rate, GFR < 45mL/min)78 be discovered during pre-operative evaluation or develop following surgery, metformin is discontinued until renal function normalizes. If discontinued pre-operatively, notice should be provided to the patient's primary care physician or endocrinologist, and alternative oral hypoglycemic therapy considered if appropriate.
Table 2.
Oral Medication Use the Day Before and Day of Surgery
| ORAL MEDICATION for ELECTIVE SURGERY | Day before surgery | Day of surgery if: 1. Normal oral intake anticipated same day AND 2. Minimally invasive surgery |
Day of surgery if: 1. Reduced post-operative oral intake OR 2. Extensive surgery, anticipated HD changes and/or fluid shifts |
|---|---|---|---|
| Secretagogues | Take | Hold | Hold |
| SGLT-2 Inhibitors | Hold | Hold | Hold |
| Thiazolidinediones | Take | Take | Hold |
| Metformin | Take§ | Take§ | Hold |
| DPP-4 Inhibitors | Take | Take | Take |
HD = Hemodynamic, SGLT = Sodium glucose cotransporter-2, DPP = Dipeptidyl peptidase 4
Hold if patient having a procedure with IV contrast dye administration, particularly in those with GFR <45 milliliters/minute78
Due to reports of diabetic ketoacidosis (DKA) occurring in conjunction with sodium glucose-cotransporter 2 (SGLT-2) inhibitor therapy, the FDA released a safety statement in 2015.76 In response to this statement, the American College of Endocrinology (ACE) and AACE convened a conference of experts to evaluate and minimize the risk of DKA in patients using SGLT-2 inhibitors.77 Recommendations from the symposium's emerging guidelines include stopping the drug in patients undergoing emergency surgery and holding the medication 24 hours before an elective surgery or invasive procedure.
There is growing interest in the incretin family (DPP-4s and GLP-1 agonists) as agents to improve glycemic control without increasing the incidence of hypoglycemia. Currently, randomized placebo-controlled trials are underway examining sitagliptan for use in both cardiac and general surgery patients with type 2 diabetes. DPP-4 inhibitors were proven to be safe in a recent randomized trial of medical and non-cardiac surgery patients with type 2 diabetes treated at home with diet, oral antidiabetic agents, or a low daily insulin dose (≤0.4 units/kg/day).54 DPP-4 inhibitors may be taken the day of surgery and continued into the perioperative period.
Patients with type 2 diabetes treated with insulin should continue their insulin therapy (Table 3). It has been suggested that the patient's basal insulin (glargine or detemir) dose be reduced by approximately 25% of normal dose the evening before55 or morning of surgery if twice daily dosing. NPH insulin and premixed formulations are reduced by 20% the evening before surgery and by 50% the morning of surgery.56 In addition, we recommend holding the dose of NPH or premixed insulin the morning of surgery in patients with type 2 diabetes and fasting glucose < 120 mg/dl. Day of surgery regimens are outlined in Table 4.
Table 3.
Day before surgery insulin regimens based on oral intake status
| DAY BEFORE SURGERY INSULIN REGIMENS | Glargine or Detemir | NPH or 70/30 insulin | Lispro, aspart, glulisine, regular | Non-Insulin Injectables | ||||
|---|---|---|---|---|---|---|---|---|
| AM Dose | PM Dose | AM Dose | PM Dose | AM Dose | PM Dose | AM Dose | PM Dose | |
| Normal Diet until Midnight (includes those permitted clear liquids until 2hrs prior to surgery) | Usual dose | 80%% of usual dose | 80% of usual dose | 80% of usual dose | Usual dose | Usual dose | Usual dose | Usual dose |
| Bowel Prep (and/or clear liquids only 12-24hrs prior to surgery) | Usual dose | 80% of usual dose | 80% of usual dose | 80% of usual dose | Usual dose | Usual dose | Hold when starting clear liquid diet/bowel prep | Hold when starting clear liquid diet/bowel prep |
Table 4.
Day of Surgery Insulin Regimens
| DAY OF SURGERY INSULIN REGIMENS | Glargine or Detemir | NPH or 70/30 insulin | Lispro, aspart, glulisine, regular | Non-insulin injectables |
| 80% of usual dose if patient uses twice daily basal therapy | 50% of usual dose if BG 120mg/dL* Hold for BG < 120mg/dL |
Hold | Hold |
BG = Blood Glucose
6.6mmol/L
Patients with type 1 diabetes undergoing surgical procedures require insulin during the perioperative period. The stress of surgery may result in severe hyperglycemia or ketoacidosis. These patients should receive 80% of basal insulin dose the evening before surgery and on the morning of surgery in order to prevent hypoglycemia.57 Prandial insulin is stopped when the fasting state begins.
Intraoperative Glycemic Management
The target perioperative blood glucose level depends upon the duration of surgery, invasiveness of surgical procedure, type of anesthetic technique, and expected time to resume oral intake and routine antidiabetic therapy. The Endocrine Society and SAMBA recommend that intraoperative blood glucose levels be maintained <180 mg/dL.30,48
Hyperglycemia (>180 mg/dL, 10 mmol/l) is treated with subcutaneous (SC) rapid-acting insulin analogs or with an intravenous infusion of regular insulin. Patients undergoing ambulatory surgery or procedures of short duration (< 4 hours operating room time) are often appropriate candidates for SC insulin treatment. Additionally, SC rapid-acting insulin analogues may also be used to correct hyperglycemia during inpatient procedures that are minimally invasive, with expected hemodynamic stability, and allow early resumption of oral intake.48,53 Onset time of rapid-acting insulin analogs is between 15-30 minutes with peak drug effect occurring between 1-1.5 hours. Advantages of SC rapid-acting insulin analogs include ease of administration, low rate of hypoglycemia, and efficacy in correcting hyperglycemia.58
When subcutaneous insulin is used in the preoperative period or operating room to treat hyperglycemia, BG testing should occur at least every two hours. Correctional insulin is defined as the supplemental insulin provided for BG > 180mg/dL (10 mmol/L) following blood glucose testing. Correctional dosing with a rapid-acting insulin can be calculated with the following formula: Measured glucose – 100 / insulin sensitivity factor. Insulin sensitivity factor is equal to 1800 divided by the patient's total daily dose of insulin. The total daily dose (TDD) is equivalent to the patient's daily amount of basal, prandial and correctional insulin. Should TDD not be available or if a patient is using only oral medications at home, a sensitivity factor (denominator) of 40 provides a safe calculation for a correctional insulin dose. Rapid-acting insulin should not be dosed more frequently than every two hours to minimize the risk of insulin stacking. Data comparing SC insulin to intravenous insulin infusion in the operative setting is lacking. The short duration of action of rapid-acting insulin analoges limits the risk of ‘insulin stacking’ with repeat dosing. However, limiting the number of intraoperative SC insulin doses to two, within the four-hour operative time, may reduce the risk hypoglycemia.
Algorithms are available that recommend SC insulin dosing regimens to treat intraoperative hyperglycemia.59 Table 5 provides a SC rapid-acting insulin correction scale that can be used intra and post-operatively. To limit the risk of hypoglycemia, patient factors associated with insulin sensitivity (age > 70 years, renal insufficiency) are identified, and a reduced correctional dose is provided for BG > 180mg/dL (10mmol/L). Larger doses of correctional SC insulin are provided for BG > 180mg/dL (10 mmol/L) in patients with factors associated with insulin resistance (BMI > 35kg/m2, TDD > 80U/day, steroid equivalent > 20mg prednisone daily). Should a patient have factors in multiple categories (insulin sensitive, insulin usual, insulin resistant), the category with smallest correctional dose is chosen to minimize the risk of hypoglycemia.
Table 5.
Correctional SC Insulin Scale Day of Surgery and Post-Operative Surgical Ward Care
| Blood Glucose mg/dL (mmol/L) |
Insulin Sensitive* Age > 70, GFR < 45mL/min, No history of diabetes |
Usual Insulin | Insulin Resistant* BMI > 35kg/m2, Home TDD Insulin > 80U, Steroids > 20mg prednisone daily |
|---|---|---|---|
| 141-180 (7.7-10) | 0 | 2 | 3 |
| 181-220 (10-12.2) | 2 | 3 | 4 |
| 221-260 (12.2-14.4) | 3 | 4 | 5 |
| 261-300 (14.4-16.6) | 4 | 6 | 8 |
| 301-350 (16.6-19.4) | 5 | 8 | 10 |
| 351-400 (19.4-22.2) | 6 | 10 | 12 |
| > 400 (> 22.2) | 8 | 12 | 14 |
(GFR: glomerular filtration rate, mL: milliliter, min: minute, m: meters, U: units, kg: kilograms, BMI: body mass index, mg: milligrams, d: deciliter, mmol: millimoles, L: liter, TDD: total daily dose)
If the patient falls into more than one insulin treatment group, choose the category with the lowest correctional dose to minimize the risk of hypoglycemia.
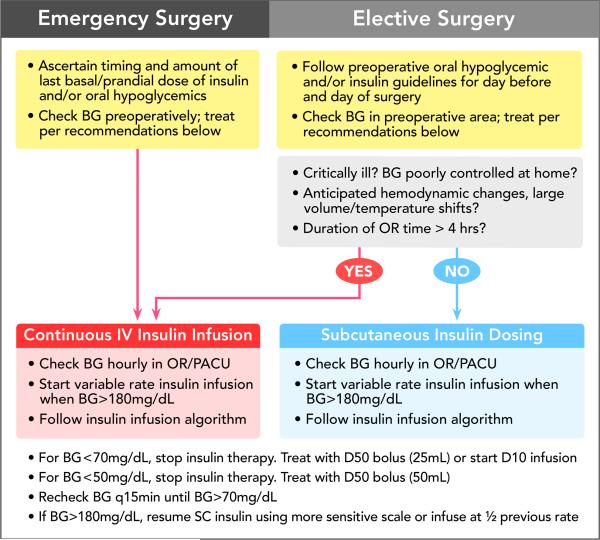
An intravenous (IV) insulin infusion is recommended in patients undergoing procedures with anticipated hemodynamic changes, significant fluid shifts, expected changes in temperature (passive hypothermia or active cooling, hyperthermic intraperitoneal chemotherapy), use of inotropes, or lengthy operative times (>4 hours). These variables alter subcutaneous insulin absorption and distribution. Unreliable pharmacokinetics may result in persistent hyperglycemia or conversely, sudden hypoglycemia. For these same reasons, an IV insulin infusion is used in critically ill patients or those undergoing cardiac surgery. The short half-life of IV insulin (less than 15 minutes) allows for rapid-adjustment in drug delivery and has limited lasting effect. The ideal protocol allows flexible rate adjustments that are based on both the current and previous glucose value. An example is provided in Table 6. Blood glucose testing should occur hourly when using a variable rate insulin infusion. Given the frequency of monitoring, use of an electronic alert system in the operating room can increase provider adherence to testing and treatment of blood glucose.60 A pre and intraoperative testing and treatment algorithm is outlined in Figure 2.
TABLE 6. Variable Rate Continuous Insulin Infusion.
Perioperative Target Blood Glucose 140-180mg/dL (7.8-10mmol/L)
| Blood Glucose mg/dL (mmol/L) | If BG increased from previous measurement | BG decreased from prior measurement by less than 30mg/dL | BG decreased from prior measurement by greater than 30mg/dL |
|---|---|---|---|
| > 241 (13.4) | Increase rate by 3U/h | Increase rate by 3U/h | No change in rate |
| 211-240 (11.7-13.4) | Increase rate by 2U/h | Increase rate by 2U/h | No change in rate |
| 181-210 (10-11.7) | Increase rate by 1U/h | Increase rate by 1U/h | No change in rate |
| 141-180 (7.8-10) | No change in rate | No change in rate | No change in rate |
| 110-140 (6.1-7.8) | No change in rate | Decrease rate by ½ U/h | Hold insulin infusion |
| 100-109 (5.5-6.1) | 1. Hold insulin infusion 2. Re-check BG hourly 3. Restart infusion at ½ the prior infusion rate if BG > 180mg/dL (10mmol/L) |
||
| 71-99 (3.9-5.5) | 1. Hold insulin infusion 2. Check BG every 30 minutes until BG > 100mg/dL (5.5mmol/L) 3. Resume BG checks every hour 4. Restart infusion at ½ the prior infusion rate if BG > 180mg/dL (10mmol/L) |
||
| 70 (3.9) or lower | If BG 50-70 (2.8-3.9mmol/L), 1. Give 25mL D50 2. Repeat BG checks every 30 minutes until BG > 100mg/dL (5.5mmol/L) If BG < 50mg/dL (2.8mmol/L) 1. Give 50mL D50 2. Repeat BG every 15 minutes until > 70mg/dL (3.9mmol/L) 3. When BG > 70mg/dL, BG checks every 30 minutes until > 100mg/dL (5.5mmol/L). Repeat 50mL D50 dose if BG < 50mg/dL a second time and start D10 infusion. 4. After BG > 100mg/dL (5.5mmol/L), resume hourly BG check Restart infusion at ½ the prior infusion rate if BG > 180mg/dL (5.5mmol/L) |
||
BG: Blood Glucose, mg: milligrams, dL: deciliter, mmol: millimoles, L: liter, U: Units, h: hour, D50: 50% dextrose solution, D10: 10% dextrose solution, mL: milliliters
1. If BG > 180mg/dL (10mmol/L), start insulin infusion
2. Consider bolus dose [BG – 100/40]
3. Start rate at BG/100 = U/hr
4. Check BG hourly and correct per table
Figure 2.
Pre and Intraoperative Testing and Treatment Algorithm (Intravenous or Subcutaneous Insulin)
(NPO: nothing by mouth, po: oral intake, BG: blood glucose, OR: operating room, PACU: post anesthesia care unit, q2h: every 2 hours, q30min: every 30 minutes, q15min: every 15 minutes, D50: Dextrose 50% Solution, D10: Dextrose 10% Solution, SC: subcutaneous, mg: milligrams, d: deciliter, mmol: millimoles, L: liter)
BG 180mg/dL = 10mmol/L, BG 140mg/dL = 7.8mmol/L, 70mg/dL = 3.9mmol/L, BG 50mg/dL = 2.8mmol/L
Post-Operative Glycemic Management for non-ICU Patients
Glucose control in non-critically ill, non-ICU surgical patients is managed with SC insulin. While recovering in the post-anesthesia care unit (PACU), BG checks need to continue at least every two hours for all diabetic patients, and for non-diabetics treated with insulin in the operating room. Correctional SC rapid-acting insulin doses are provided for BG > 180mg/dL (10mmol/L) (Table 5). The anesthesiologist's assessment of the patient's level of consciousness, recovery condition, ability to swallow, and oral status determined by surgical team are used to determine treatment of BG < 70mg/dL (3.9mmol/L). An oral glucose or IV dextrose solution is appropriate. If a PACU patient's condition deteriorates and there is need for ICU level care, all SC insulin is stopped and an IV insulin drip started for BG > 180mg/dL (10mmol/L) (Table 6). For same-day surgery, the patient may resume their home medication regimen when he/she leaves the hospital.
When patients transition out of the PACU onto the surgical floor, use of sliding scale insulin alone is not acceptable as the single regimen in patients with diabetes, as it results in undesirable hypoglycemia and/or hyperglycemia.61,62 The administration of once or twice daily basal insulin (glargine, determir, NPH) alone, or in combination with prandial insulin is the recommended approach.61-63 The use of a long-acting basal insulin plus prandial rapid-acting insulin is commonly referred to as a “Basal Bolus” insulin regimen. The RABBIT 2 trial reported that in general medicine patients with type 2 diabetes, basal-bolus treatment resulted in greater control of blood glucose than regimens consisting only of sliding-scale insulin.61 In general surgery patients, basal-bolus regimens also significantly reduce the number of postoperative complications, primarily wound infections.62 NPH and regular insulin, or premixed (70/30) formulations, demonstrate equivalent blood glucose control when compared to basal bolus regimens, but are associated with higher rates of hypoglycemia in patients with poor oral intake.64,65
The “Basal Plus” regimen includes a long-acting basal insulin once daily plus a correctional rapid-acting insulin to treat BG > 180mg/dL.63 Although the correctional insulin appears similar to a sliding scale, it is intended to make small corrections in BG that occur despite basal therapy. Sliding scale insulin regimens do not supply basal insulin. In surgical patients with reduced total caloric intake, the Basal Plus trial63 reported that a single daily dose of glargine plus correctional doses with rapid-acting insulin resulted in improved glycemic control compared to sliding scale insulin therapy alone. There was no difference in the frequency of hypoglycemia compared to a basal bolus regimen. These results indicate that in surgical patients, the basal plus correctional insulin regimen is preferred for patients with poor or no oral intake while an insulin regimen with basal, prandial and correctional components (Basal Plus) is preferred for patients with good nutritional intake.
Insulin doses administered subcutaneously are calculated either based on weight or on home insulin doses (Table 7). For insulin naïve diabetic patients, an evaluation of oral intake determines basal daily dose. For patients who are NPO or with poor oral intake, the starting daily dose of basal (glargine, detemir) insulin is 0.2 to 0.25 units.kg−1.day−1. As patients’ diet orders are advanced and they tolerate a diet (regular, low carbohydrate or diabetic), a basal bolus regimen is started. In elderly patients (age > 70) and those with impaired renal function (GRF < 45ml/min), the daily basal insulin dose is reduced by approximately half of the normal recommended dose (0.1 to 0.15 units.kg−1.day−1) to reduce the risk of hypoglycemia. Insulin resistant patients are provided higher doses of basal insulin (0.3 units.kg−1.day−1) to minimize hyperglycemia. A correctional scale (Table 5) is included for all diabetic patients in both the basal plus and basal bolus regimen.
Table 7.
Post-Operative Surgical Floor Insulin for Type 2 Diabetics on Oral Agents at Home
| Type of Insulin | TOTAL DAILY DOSE Insulin Sensitive* Age > 70 years, GFR < 45mL/min |
TOTAL DAILY DOSE Insulin Usual |
TOTAL DAILY DOSE Insulin Resistant* BMI > 35 kg/m2, Steroids≥ 20mg prednisone daily |
|
|---|---|---|---|---|
|
NPO/Poor Oral Intake/Clear Liquid Diet
BASAL PLUS REGIMEN |
BASAL (Glargine/Detemir) | 0.1 - 0.15 U•kg−1 •day−1 | 0.2-0.25 U•kg−1 •day−1 | 0.3 U•kg−1 •day−1 |
| CORRECTIONAL (Rapid-Acting) | Test BG every six hours. Treat BG > 180mg/dL (10mmol/L) using correctional calculation or Table 5 |
|||
|
Normal oral intake at meal time
BASAL BOLUS REGIMEN |
BASAL (Glargine/Detemir) | 0.1 – 0.15 U•kg−1 •day−1 | 0.2 - 0.25 U•kg−1 •day−1 | 0.3 U•kg−1 •day−1 |
| PRANDIAL (Lispro/Aspart) | 0.1 - 0.15 U•kg−1 •day−1 | 0.2 – 0.25 U•kg−1 •day−1 | 0.3 U•kg−1 •day−1 | |
| Give 1/3 prandial insulin with each meal | ||||
| CORRECTIONAL (Rapid-Acting) | Test BG with meals and at bedtime. Treat BG > 180mg/dL (10mmoL/L) |
|||
(GFR: glomerular filtration rate,mL: milliliter, min: minute, BMI: body mass index, m: meters, kg: BG: blood glucose, dL: deciliter, mg: milligrams, mmol: millimoles, L: liter)
If the patient falls into more than one insulin treatment group, choose the category with the lowest insulin dose to minimize the risk of hypoglycemia.
The home insulin regimen of a diabetic patient is used to calculate daily hospital dose. Reducing TDD by 20-25% yields the starting inpatient dose of basal insulin while the patient is NPO. The reduction in basal insulin reduces the risk of hypoglycemia, particularly in those with poor or uncertain caloric intake.63 The dose of basal insulin is adjusted daily if the patient's blood glucose is not within target range over the previous 24 hours. In the absence of hypoglycemia (BG < 70mg/dL, 3.8mmol/L), the basal insulin dose is increased by 10 or 20% respectively for persistent BG > 180mg/dl (10mmol/L) or 240mg/dl (13mmol/L). As normal diet is resumed, insulin therapy can be transitioned to the patient's usual basal and prandial regimen. An endocrinology consult is recommended in diabetic patients if they are started, or placed on increasing doses of steroids or immunosuppresants, if parenteral or enteral feeding is initiated (or with formula change), for persistent hypoglycemia or hyperglycemia (despite dose adjustment) and before discharge if the patient's home regimen is not controlling their disease (HgbA1C > 8%).
The use of oral antidiabetic agents is generally not recommended in hospitalized patients due to the limited data available on their safety and efficacy. Hospitalized patients frequently have contraindications to oral medications and the slow onset of action may preclude achieving rapid glycemic control. However, in recent years, the use of DPP-4 inhibitors has been proposed for the management of inpatient hyperglycemia.54 A recent randomized study in medicine and surgery patients with type 2 diabetes reported that the use of sitagliptin alone, or in combination with a single basal insulin dose, resulted in similar mean daily glucose concentrations when compared to basal bolus insulin regimens.54
Transitioning from IV to SC insulin
Post-operatively, glycemic control in critically ill patients is managed with a continuous insulin infusion. When ICU patients are ready to be transferred to the general medical floors, appropriate transition orders from an IV insulin to scheduled SC insulin are needed to prevent rebound hyperglycemia.66 This is imperative in patients with type 1 diabetes since stopping or delaying insulin for only a few hours can result in diabetic ketoacidosis.
Calculation of SC insulin dose in those who have been on an IV insulin infusion is done by determining the TDD of insulin based on the patient's insulin infusion over the last 8 hours. Seventy percent of this total is administered as basal insulin. Thirty percent is added as prandial insulin when the patient is tolerating a normal diet.67 For diabetic patients on insulin therapy before admission, surgical ward insulin dose is based on home regimen.63,66 Reducing the patient's home TDD of insulin by 20-25%, provides the starting daily basal insulin dose for the patient while NPO or with limited oral intake. To prevent rebound hyperglycemia, basal insulin is given 2 hours before discontinuation of the IV insulin infusion. Patients without a history of diabetes (HbA1c < 6.5%) requiring insulin infusion at low doses (≤ 2 U/hour), can be transitioned to the surgical floor without basal insulin. Continued monitoring is necessary and correctional insulin may be needed.25
Insulin Pump Therapy
The use of insulin pump therapy has increased significantly during the past decade with recent estimates of more than 400,000 pump users in the United States.68 The majority of patients with an insulin pump have type 1 diabetes but, use is growing in type 2 diabetics. Patients with an insulin pump should continue insulin preoperatively and on the day of surgery. Depending on provider comfort levels managing an insulin pump and its settings, as well the placement of the pump in relation to the surgical field, a patient's home device may be used in the operating room. Advanced pre-operative planning to facilitate pump use in the operating room, especially for shorter cases or outpatient surgery, prevents interruption of the patient's normal insulin routine.
Intraoperatively, if the patient's own insulin pump is used, the basal rate is continued. Hourly monitoring of blood glucose is initiated when the patient arrives to the pre-operative area and continued until the patient is sufficiently alert to resume self-management. The pump is turned off when the BG < 110mg/dL / 6.1mmol/L). Correctional insulin bolus therapy is provided to treat BG > 180mg/dL (10mmol/L). Alternatively, if the pump cannot be used because placement interferes with the surgical field, the anesthesia team cannot access the pump due to positioning or, if turning off and/or changing basal rate is difficult for the anesthesiology team, an IV insulin infusion can be substituted for the pump. The pump is disconnected and an insulin infusion started at the same basal rate used by the patient.
Post-operatively, successful management of inpatient diabetes with the continuation of insulin pump therapy has been demonstrated in selected patients.70 Current recommendations advocate for the establishment of clear policies and procedures to guide patients and hospital staff in the management of diabetes with the use of insulin pumps.68 Patients who are sufficiently alert to aid in their BG testing, pump rate change and bolus administration, can continue to use and manage their pump as an inpatient. In addition to the other previously mentioned reasons for consult, prompt involvement of inpatient diabetes specialists is recommended to assist with the assessment and management of critically ill or sedated/somnolent patients, or if the patient has difficulty controlling blood glucose with typical rate adjustments and prandial doses.69
Hypoglycemia
Hypoglycemia (< 70mg/dL, 3.9 mmol/l) is the most common event associated with insulin administration and has been demonstrated to be associated with poor clinical outcomes10 and mortality.7 Clear data demonstrates that the probability of hypoglycemia increases significantly when glycemic goals are aggressive.42 Because hypoglycemia may go unrecognized under anesthesia,71 providers can be reticent to use insulin in the operating room. Undoubtedly, care must be taken to appropriately monitor patients in the perioperative setting to prevent dangerous drops in blood sugar. Conservative BG targets,7 frequent monitoring, perioperative provider communication,72 and treatment algorithms that base doses on insulin sensitivity,59 jointly reduce the risk of intra and post-operative hypoglycemia.
Glucose Monitoring in the Perioperative Period
Options for testing blood glucose include central laboratory testing, blood gas analysis and capillary point of care testing (POC). Although central lab testing provides the most accurate BG measurement, the immediate turn-around time of POC glucometer devices enable anesthesia providers to make quick decisions to treat both hyper and hypoglycemia. However, intensivists, anesthesiologists and surgeons need to recognize the limitations of glucometer POC testing. In 2014, the FDA issued a draft guidance outlining that 99% of POC readings > 70mg/dL (3.9mg/dL) be within 10% of central lab reference values and that all BG readings < 70mg/dL be within 7mg/dL (0.39mmol/L). Glucometers available in many hospitals do not meet these metrics and may be less accurate than providers recognize. The safety of these devices to monitor BG in critically ill patients has been extensively questioned. A recent review of 21 studies examining POC glucose testing in this population, suggested that in the setting hemodynamic instability or continuous insulin infusions, bedside glucometers may not be sufficiently precise or reliable for use.73
Conclusion
Hyperglycemia is common in surgical patients. Current data demonstrates an association between elevated BG and a risk of perioperative complications in diabetic and non-diabetic patients. Insulin administration intra and post-operatively has been shown to improve clinical outcomes. Individual patient characteristics and surgical case factors are considered when choosing subcutaneous insulin or an insulin infusion. Both are appropriate options on the day of surgery. Blood glucose values of 180 mg/dL (10 mmol/L) or higher are treated with insulin. Target range for the perioperative period is 140-180 mg/dL (7.7-10 mmol/L) (see Table 1).48-52. Post-operatively, surgical floor patients with poor or uncertain oral intake are treated with once daily basal insulin. Prandial insulin is added when patients tolerate oral intake. Increasing evidence suggests a role for incretin therapy during the peri-operative period in patients with type 2 diabetes.
Multiple teams care for a surgical patient during the hospital course (anesthesiology, surgery, critical care medicine, internal/hospital medicine and endocrinology). Therefore, multi-disciplinary groups within an institution should work together to create appropriate protocols for hyperglycemia screening, monitoring and treatment to minimize errors and to better care for patients.
Acknowledgments
Support for this article was provided solely from institutional and/or department resources.
Dr. Umpierrez is supported in part by research grants from the American Diabetes Association (1-14-LLY-36), PHS grant UL1 RR025008 from the Clinical Translational Science Award Program (M01 RR-00039), National Institute of Health, National Center for Research Resources (1P30DK111024-01), and has received unrestricted research support for inpatient studies (to Emory University) from Merck, Novo Nordisk, Astra Zeneca, Boehringer Ingelheim, and Sanofi, and has received consulting fees or/and honoraria for membership in advisory boards from Novo Nordisk, Sanofi, Merck, Glytec, and Johnson and Johnson.
Footnotes
Conflict(s) of Interest
Drs. Duggan and Carlson declare no conflict of interest.
References
- 1.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 2.Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatfcliffe C, Hudson M, Mendoza J, Johnson R, Lin E, Umpierrez G. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33(8):1783–1788. doi: 10.2337/dc10-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotagal M, Symons RG, Hirsch IB, Umpierrez GE, Farrokhi ET, Flum DR, SCOAP-Certain Collaborative Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg. 2015;261(1):97–103. doi: 10.1097/SLA.0000000000000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrokhi F, Smiley D, Umpierrez GE. Glycemic control in non-diabetic critically ill patients. Best Pract Res Clin Endocrinol Metab. 2011;25(5):813–824. doi: 10.1016/j.beem.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 6.Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, Umpierrez D, Newton C, Olson D, Rizzo M. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256–261. doi: 10.2337/dc10-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finfer S, Chittock DR, Su SY, Mitchell I, Myburgh J, Norton R, Potter J, the NICE-SUGAR Study Investigators Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 8.NICE-SUGAR Study Investigators. Finfer S, Liu B, Chittock DR, Norton R, Myburgh JA, McArthur C, Mitchell I, Foster D, Dhingra V, Henderson WR, Ronco JJ, Bellomo R, Cook D, McDonald E, Dodek P, Hébert PC, Heyland DK, Robinson BG. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367(12):1108–1118. doi: 10.1056/NEJMoa1204942. [DOI] [PubMed] [Google Scholar]
- 9.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 10.Esposito K, Nappo F, Marfella R, Guigliano G, Guigliano F, Ciotola M, Quagliaro L, Ceriello A, Guigliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106(16):2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 11.Chan TM. The permissive effects of glucocorticoid on hepatic gluconeogenesis. Glucagon stimulation of glucose-suppressed gluconeogenesis and inhibition of 6-phosphofructo-1-kinase in hepatocytes from fasted rats. J Biol Chem. 1984;259(12):7426–7432. [PubMed] [Google Scholar]
- 12.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97(12):2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline CW, Siezak LA, Andersen DK, Hundai RS, Rothman DL, Petersen KF, Shulman GI. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103(2):253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91(11):4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorell A, Efendic S, Gutniak M, Haggmark T, Ljungqvist O. Insulin resistance after abdominal surgery. Br J Surg. 1994;81(1):59–63. doi: 10.1002/bjs.1800810120. [DOI] [PubMed] [Google Scholar]
- 16.Svanfeldt M, Thorell A, Hausel J, Soop M, Nygren J, Ljungqvist O. Effect of “preoperative” oral carbohydrate treatment on insulin action–a randomised cross-over unblinded study in healthy subjects. Clin Nutr. 2005;24:815–21. doi: 10.1016/j.clnu.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Wang ZG, Wang Q, Wang WJ, Qin HL. Randomized clinical trial to compare the effects of preoperative oral carbohydrate versus placebo on insulin resistance after colorectal surgery. Br J Surg. 2010;97:317–27. doi: 10.1002/bjs.6963. [DOI] [PubMed] [Google Scholar]
- 18.Awad S, Varadhan KK, Ljungqvist O, Lobo DN. A meta-analysis of randomised controlled trials on preoperative oral carbohydrate treatment in elective surgery. Clin Nutr. 2013;32:34–44. doi: 10.1016/j.clnu.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Clarke RS. The hyperglycaemic response to different types of surgery and anaesthesia. Br J Anaesth. 1970;42(1):45–53. doi: 10.1093/bja/42.1.45. [DOI] [PubMed] [Google Scholar]
- 20.Rehman HU, Mohammed K. Perioperative management of diabetic patients. Curr Surg. 2003;60(6):607–611. doi: 10.1016/j.cursur.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Desborough JP, Jones PM, Persaud SJ, Landon MJ, Howell SL. Isoflurane inhibits insulin secretion from isolated rat pancreatic islets of Langerhans. Br J Anaesth. 1993;71(6):873–876. doi: 10.1093/bja/71.6.873. [DOI] [PubMed] [Google Scholar]
- 22.Lattermann R, Schricker T, Wachter U, Georgieff M, Goertz A. Understanding the mechanisms by which isoflurane modifies the hyperglycemic response to surgery. Anesth Analg. 2001;93(1):121–127. doi: 10.1097/00000539-200107000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Cook CB, Kongable GL, Potter DJ, Abad VJ, Leija DE, Anderson M. Inpatient glucose control: a glycemic survey of 126 U.S. hospitals. J Hosp Med. 2009;4(9):E7–E14. doi: 10.1002/jhm.533. [DOI] [PubMed] [Google Scholar]
- 24.Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8–14. doi: 10.1097/SLA.0b013e31827b6bbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umpierrez G, Cardona S, Pasquel F, Jacobs S, Peng L, Unigwe M, Newton CA, Smiley-Byrd D, Vellanki P, Halkos M, Puskas JD, Guyton RA, Thourani VH. Randomized Controlled Trial of Intensive Versus Conservative Glucose Control in Patients Undergoing Coronary Artery Bypass Graft Surgery: GLUCO-CABG Trial. Diabetes Care. 2015;(9):1665–1672. doi: 10.2337/dc15-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swanson CM, Potter DJ, Kongable GL, Cook CB. An Update on Inpatient Glycemic Control in U.S. Hospitals. Endocr Pract. 2011:1–22. doi: 10.4158/EP11042.OR. [DOI] [PubMed] [Google Scholar]
- 27.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373(9677):1798–1807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greci LS, Kailasam M, Malkani S, Katz DL, Hulinsky I, Ahmadi R, Nawaz H. Utility of HbA(1c) levels for diabetes case finding in hospitalized patients with hyperglycemia. Diabetes Care. 2003;26(4):1064–1068. doi: 10.2337/diacare.26.4.1064. [DOI] [PubMed] [Google Scholar]
- 29.Mazurek JA, Hailpern SM, Goring T, Nordin C. Prevalence of Hemoglobin A1c Greater Than 6.5% and 7.0% among Hospitalized Patients without Known Diagnosis of Diabetes at an Urban Inner City Hospital. J Clin Endocrinol Metab. 2010;95(3):1344–1348. doi: 10.1210/jc.2009-1151. [DOI] [PubMed] [Google Scholar]
- 30.Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM, Seley JJ, Van den Berghe G, Endocrine Society Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38. doi: 10.1210/jc.2011-2098. [DOI] [PubMed] [Google Scholar]
- 31.Abdelmalak BB, Knittel J, Abdelmalak JB, Dalton JE, Christiansen E, Foss J, Argalious M, Zimmerman R, Van den Berghe G. Preoperative blood glucose concentrations and postoperative outcomes after elective non-cardiac surgery: an observational study. Br J Anaesth. 2014;112(1):79–88. doi: 10.1093/bja/aet297. [DOI] [PubMed] [Google Scholar]
- 32.Han HS, Kang SB. Relations between long-term glycemic control and postoperative wound and infectious complications after total knee arthroplasty in type 2 diabetics. Clin Orthop Surg. 2013;5(2):118–123. doi: 10.4055/cios.2013.5.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dronge AS, Perkal MF, Kancir S, Concato J, Aslan M, Rosenthal RA. Long-term glycemic control and postoperative infectious complications. Arch Surg. 2006;141(4):375–380. doi: 10.1001/archsurg.141.4.375. discussion 380. [DOI] [PubMed] [Google Scholar]
- 34.Halkos ME, Lattouf OM, Puskas JD, Kligo P, Cooper WA, Guyton RA, Thourani VH. Elevated preoperative hemoglobin A1c level is associated with reduced long-term survival after coronary artery bypass surgery. Ann Thorac Surg. 2008;86(5):1431–1437. doi: 10.1016/j.athoracsur.2008.06.078. [DOI] [PubMed] [Google Scholar]
- 35.Gosmanov AR, Umpierrez GE. Management of hyperglycemia during enteral and parenteral nutrition therapy. Curr Diab Rep. 2013;13(1):155–162. doi: 10.1007/s11892-012-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elia M, Ceriello A, Laube H. Enteral nutritional support and use of diabetes specific formulas for patients with diabetes: a systematic review and meta-analysis. Diabetes Care. 2005;28(9):2267–2279. doi: 10.2337/diacare.28.9.2267. [DOI] [PubMed] [Google Scholar]
- 37.Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, Williams BA, Schrader LM, Rizza RA, McMahon MM. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc. 2005;80(7):862–866. doi: 10.4065/80.7.862. [DOI] [PubMed] [Google Scholar]
- 38.Fish LH, Weaver TW, Moore AL, Steel LG. Value of postoperative blood glucose in predicting complications and length of stay after coronary artery bypass grafting. Am J Cardiol. 2003;92(1):74–76. doi: 10.1016/s0002-9149(03)00472-7. [DOI] [PubMed] [Google Scholar]
- 39.Ghandi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, O'Brien PC, Johnson MG, Williams AR, Cutshall, Mundy LM, Rizza RA, McMahon MM. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146(4):233–43. doi: 10.7326/0003-4819-146-4-200702200-00002. [DOI] [PubMed] [Google Scholar]
- 40.Hua J, Chen G, Li H, Fu S, Zhang L, Scott M, Li Q. Intensive intraoperative therapy versus conventional insulin therapy during cardiac surgery: a meta-analysis. J Cardiotorac Vasc Anesth. 2012;26(5):829–34. doi: 10.1053/j.jvca.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weller N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K, German Competence Netwrok Sepsis (SepNet) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 42.Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180(8):821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desai SP, Henry LL, Holmes SD, Hunt SL, mArtin CT, Hebsur S, Ad N. Strict versus liberal target range for perioperative glucose in patients undergoing coronary artery bypass grafting: a prospective randomized controlled trial. J Thorac Cardiovasc Surg. 2012;143(2):318–325. doi: 10.1016/j.jtcvs.2011.10.070. [DOI] [PubMed] [Google Scholar]
- 44.Pezzella AT, Holmes SD, Pritchard G, Speir AM, Ad N. Impact of perioperative glycemic control strategy on patient survival after coronary bypass surgery. Ann Thorac Surg. 2014;98(4):1281–1285. doi: 10.1016/j.athoracsur.2014.05.067. [DOI] [PubMed] [Google Scholar]
- 45.Lazar HL, McDonnell MM, Chipkin S, Fitzgerald C, Bliss C, Cabral H. Effects of aggressive versus moderate glycemic control on clinical outcomes in diabetic coronary artery bypass graft patients. Ann Surg. 2011;254(3):458–463. doi: 10.1097/SLA.0b013e31822c5d78. discussion 463-454. [DOI] [PubMed] [Google Scholar]
- 46.Blaha J, Mraz M, Kopecky P, Sritesky M, Lips M, Matias M, Kunstyr J, Porizka M, Kotulak T, Kolikova I, Simanovska B, Zakharchenko M, Rulisek J, Sachl R, Anyz J, Novak D, Linder J, Hovorka R, Svacina S, Haluzik M. Perioperative Tight Glucose Control Reduces Postoperative Adverse Events in Nondiabetic Cardiac Surgery Patients. J Clin Endocrinol Metab. 2015;100(8):3081–3089. doi: 10.1210/jc.2015-1959. [DOI] [PubMed] [Google Scholar]
- 47.Pomposelli JJ, Baxter JK, 3rd, Babineau TJ, Pomfret EA, Driscoll DF, Forse RA, Bistrian BR. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22(2):77–81. doi: 10.1177/014860719802200277. [DOI] [PubMed] [Google Scholar]
- 48.Joshi GP, Chung F, Vann MA, Ahmad S, Gan TJ, Goulson DT, Merrill DG, Twersky R, Society for Ambulatory Anesthesia Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378–1387. doi: 10.1213/ANE.0b013e3181f9c288. [DOI] [PubMed] [Google Scholar]
- 49.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353–369. doi: 10.4158/EP09102.RA. [DOI] [PubMed] [Google Scholar]
- 50.Jacobi J, Bircher N, Krinsley J, Agus, Braithwaite SS, Deutschman C, Freire AX, Geehan D, Kohl B, Nasraway SA, Rigby M, Sands K, Schallom L, Taylor B, Umpierrez G, Mazuski J, Schunemann H. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40(12):3251–3276. doi: 10.1097/CCM.0b013e3182653269. [DOI] [PubMed] [Google Scholar]
- 51.Lazar HL, McDonnell M, Chipkin SR, Furnary AP, Engelman RM, Sadhu AR, Bridges CR, Haan CK, Svedjholm R, Taegtmeyer H, Shemin RJ. The Society of Thoracic Surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87(2):663–669. doi: 10.1016/j.athoracsur.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 52.Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;154(4):260–267. doi: 10.7326/0003-4819-154-4-201102150-00007. [DOI] [PubMed] [Google Scholar]
- 53.Dhatariya K, Levy N, Kilvert A, Watson B, Cousins D, Flanagan D, Hilton L, Jairam C, Leyden K, Lipp A, Lobo D, Sinclair-Hammersley M, Rayman G, Joint British Diabetes Societies NHS Diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med. 2012;29(4):420–433. doi: 10.1111/j.1464-5491.2012.03582.x. [DOI] [PubMed] [Google Scholar]
- 54.Umpierrez GE, Gianchandani R, Smiley D, Jacobs S, Wesorick DH, Newton C, Farrokhi F, Peng L, Reyes D, Lathkar-Pradhan S, Pasquel F. Safety and Efficacy of Sitagliptin Therapy for the Inpatient Management of General Medicine and Surgery Patients With Type 2 Diabetes: A pilot, randomized, controlled study. Diabetes Care. 2013;36(11):3430–3435. doi: 10.2337/dc13-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenblatt SI, Dukatz T, Jahn R, Ramsdell C, Sakharova A, Henry M, Arndt-Mutz M, Miller V, Rogers K, Balasubramaniam M. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610–617. doi: 10.1016/j.jclinane.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 56.Likavec A, Moitra V, Greenberg J, Drum M, Sweitzer BJ. Comparison of preoperative blood glucose levels in patients receiving different insulin regimens. Anesthesiology. 2006;105:A567. [Google Scholar]
- 57.Mendez CE, Umpierrez G. Management of the hospitalized patient with type I diabetes mellitus. Hosp Pract. 2013;41(4):89–100. doi: 10.3810/hp.2013.08.1072. [DOI] [PubMed] [Google Scholar]
- 58.Cook CB, Castro JC, Schmidt RE, Gauthier SM, Whitaker MD, Roust LR, Argueta R, Hull BP, Zimmrman RS. Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum. J Hosp Med. 2007;2(4):203–211. doi: 10.1002/jhm.188. [DOI] [PubMed] [Google Scholar]
- 59.Duggan EW, Klopman MA, Berry AJ, Umpierrez G. The Emory University perioperative algorithm for the management of hyperglycemia and diabetes in non-cardiac surgery. Curr Diab Rep. 2016:16. doi: 10.1007/s11892-016-0720-z. Article 34. [DOI] [PubMed] [Google Scholar]
- 60.Sathishkumar S, Lai M, Picton P, Kheterpal S, Morris M, Shanks A, Ramachandran SK. Behavioral modification of intraoperative hyperglycemia with a novel real-time audiovisual monitor. Anesthesiology. 2015;123(1):29–37. doi: 10.1097/ALN.0000000000000699. [DOI] [PubMed] [Google Scholar]
- 61.Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, Puig A, Mejia R. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181–2186. doi: 10.2337/dc07-0295. [DOI] [PubMed] [Google Scholar]
- 62.Umpierrez G, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, Umpierrez D, Newton C, Olson D, Rizzo M. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 Surgery). Diabetes Care. 2011;34(2):256–261. doi: 10.2337/dc10-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Umpierrez GE, Smiley D, Hermayer K, Khan A, Olson DE, Newton C, Jacobs S, Rizzo M, Peng L, Reyes D, Pinzon I, Fereira ME, Hunt V, Gore A, Toyoshima MT, Fonseca VA. Randomized study comparing a Basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169–2174. doi: 10.2337/dc12-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Umpierrez GE, Hor T, Smiley D, Temponi A, Umpierrez D, Ceron M, Munoz C, Newton C, Peng L, Baldwin D. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(2):564–569. doi: 10.1210/jc.2008-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bellido V, Suarez L, Rodriguez MG, Sanchez C, Dieguez M, Riestra M, Casal F, Delgado E, Menendez E, Umpierrez GE. Comparison of Basal-Bolus and Premixed Insulin Regimens in Hospitalized Patients With Type 2 Diabetes. Diabetes Care. 2015;38(12):2211–2216. doi: 10.2337/dc15-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmeltz LR, DeSantis AJ, Schmidt K, O-Shea-Mahler E, Rhee C, Brandt S, Peterson S, Molitch ME. Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia. Endocr Pract. 2006;12(6):641–650. doi: 10.4158/EP.12.6.641. [DOI] [PubMed] [Google Scholar]
- 67.Smiley D, Umpierrez GE. Management of hyperglycemia in hospitalized patients. Ann N Y Acad Sci. 2011;1212:1–11. doi: 10.1111/j.1749-6632.2010.05805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heinemann L, Fleming GA, Petrie JR, Holl RW, Bergenstal RM, Peters AL. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting, and research needs: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care. 2015;38(4):716–722. doi: 10.2337/dc15-0168. [DOI] [PubMed] [Google Scholar]
- 69.Cook CB, Boyle ME, Cisar NS, Miller-Cage V, Bourgeois P, Roust LR, Smith SA, Zimmerman RS. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital setting: proposed guidelines and outcome measures. Diabetes Educ. 2005;31(6):849–857. doi: 10.1177/0145721705281563. [DOI] [PubMed] [Google Scholar]
- 70.Bailon RM, Partlow BJ, Miller-Cage V, Boyle ME, Castro JC, Bourgeois PB, Cook CB. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract. 2009;15(1):24–29. doi: 10.4158/EP.15.1.24. [DOI] [PubMed] [Google Scholar]
- 71.Akhtar S, Barash P, Inzucchi S. Scientific principles and clinical implications of perioperative glucose regulation and control. Anes Analg. 2010;110(2):478–497. doi: 10.1213/ANE.0b013e3181c6be63. [DOI] [PubMed] [Google Scholar]
- 72.Schwenk ES, Mraovic B, Maxwell RP, Kim GS, Ehrenfeld JM, Epstein RH. Root causes of intraoperative hypoglycemia: a case series. J Clin Anesth. 2012;24(8):625–630. doi: 10.1016/j.jclinane.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Inoue S, Egi M, Kotani J. Accuracy of blood glucose measurements using glucose meters and arterail blood gas analyzers in critically-ill patients: a systematic review. Crit Care. 2013;17:R48. doi: 10.1186/cc12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Atkins J, Smith D. A review of perioperative glucose control in the neurosurgical population. J Diabetes Sci Technol. 2009;3(6):1352–1364. doi: 10.1177/193229680900300615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davis MC, Ziewacz JE, Sullivan SE, El-Sayed AM. Preoperative hyperglycemia and complication risk forllowing neurosurgical intervention: A study of 918 cases. Surg Neurol Int. 2012;3(49) doi: 10.4103/2152-7806.96071. doi: 10.4103/2152-7806.96071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.U.S. Food and Drug Administration . FDA Drug Safety Communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. U.S. Food and Drug Administration; Bethesda, MD: 2015. [August 28th, 2016]. Available at: http://www.fda.gov/drugs/drugsafety/ucm446845.htm. [Google Scholar]
- 77.Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, Ferrannini E, Fonseca VA, Garber AJ. American Association of Clinical Endocrinologists and American College of Endocrinology position statement of the association of SGLT-2 inhibitors and diabetic ketoacidosis. AACE/ACE Position Statement. Endocr Prac. 2016;22(6):753–762. doi: 10.4158/EP161292.PS. [DOI] [PubMed] [Google Scholar]
- 78.U.S. Food and Drug Administration . FDA Drug Safety Communication: FDA revises warnings regarding used of the diabetes medicine metformin in certain patients with reduced kidney function. U.S. Food and Drug Administration; Bethesda, MD: 2016. [October 22, 2016]. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm493244.htm. [Google Scholar]