Abstract
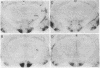
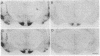
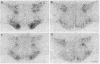
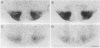
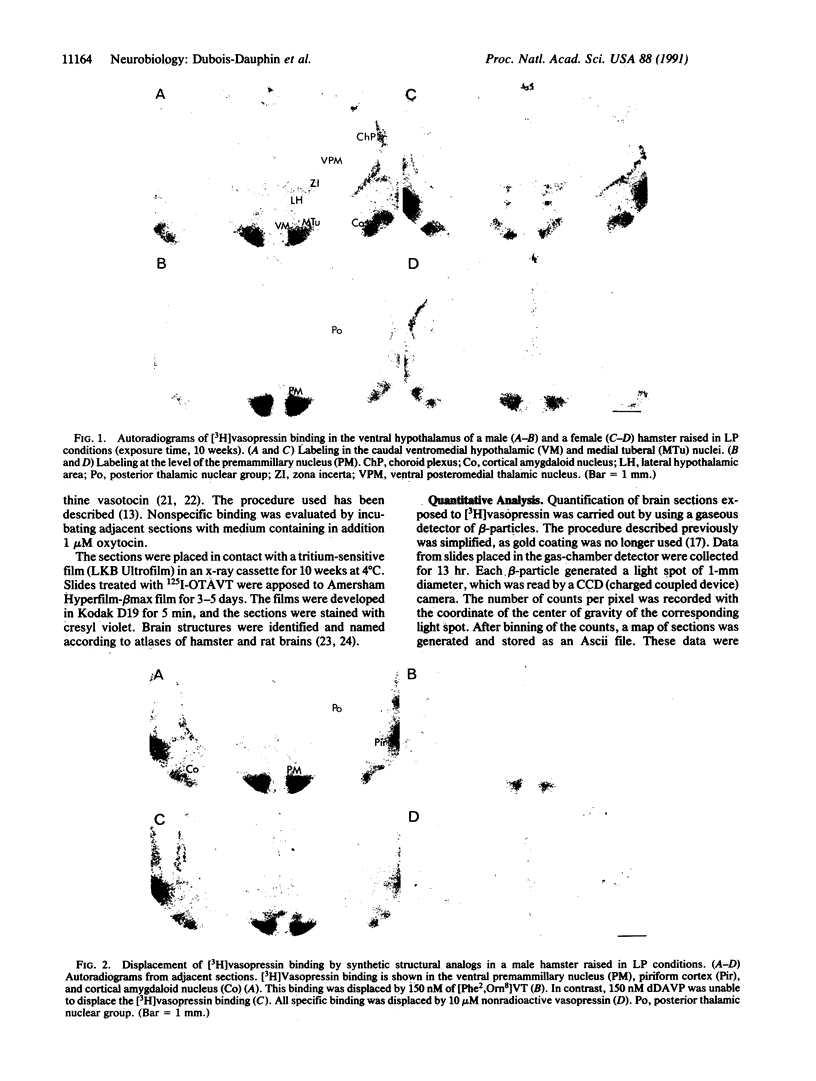
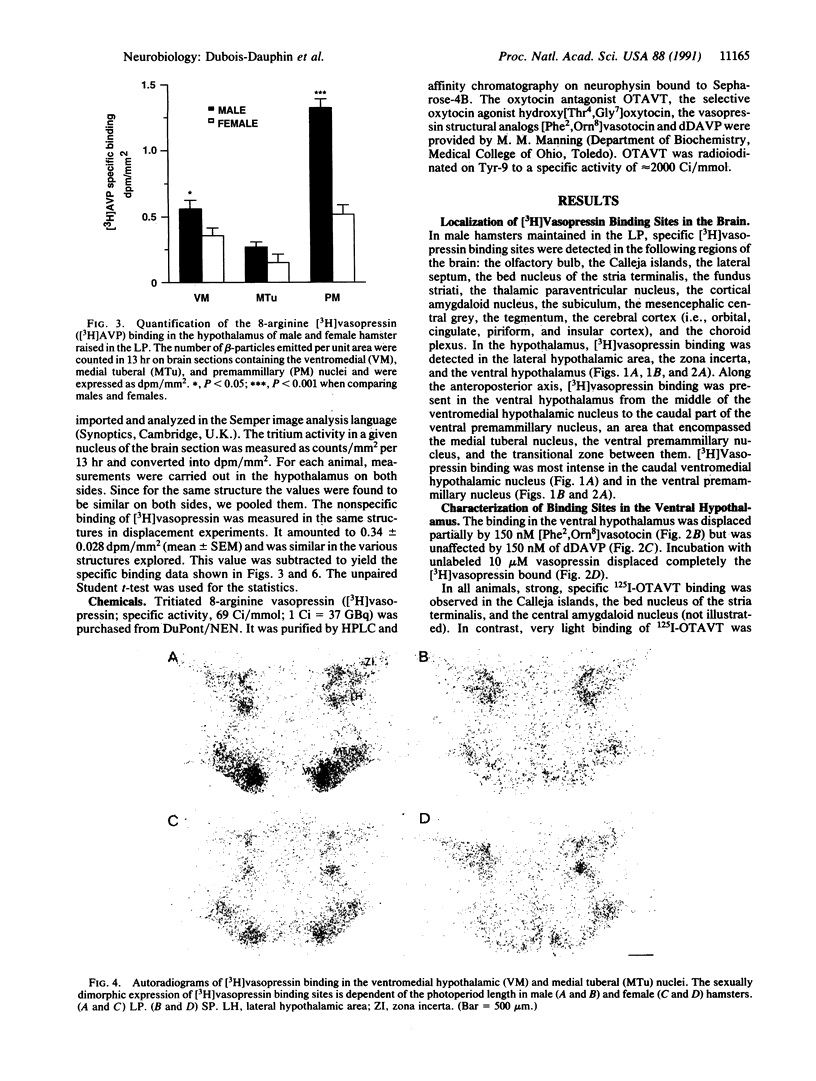
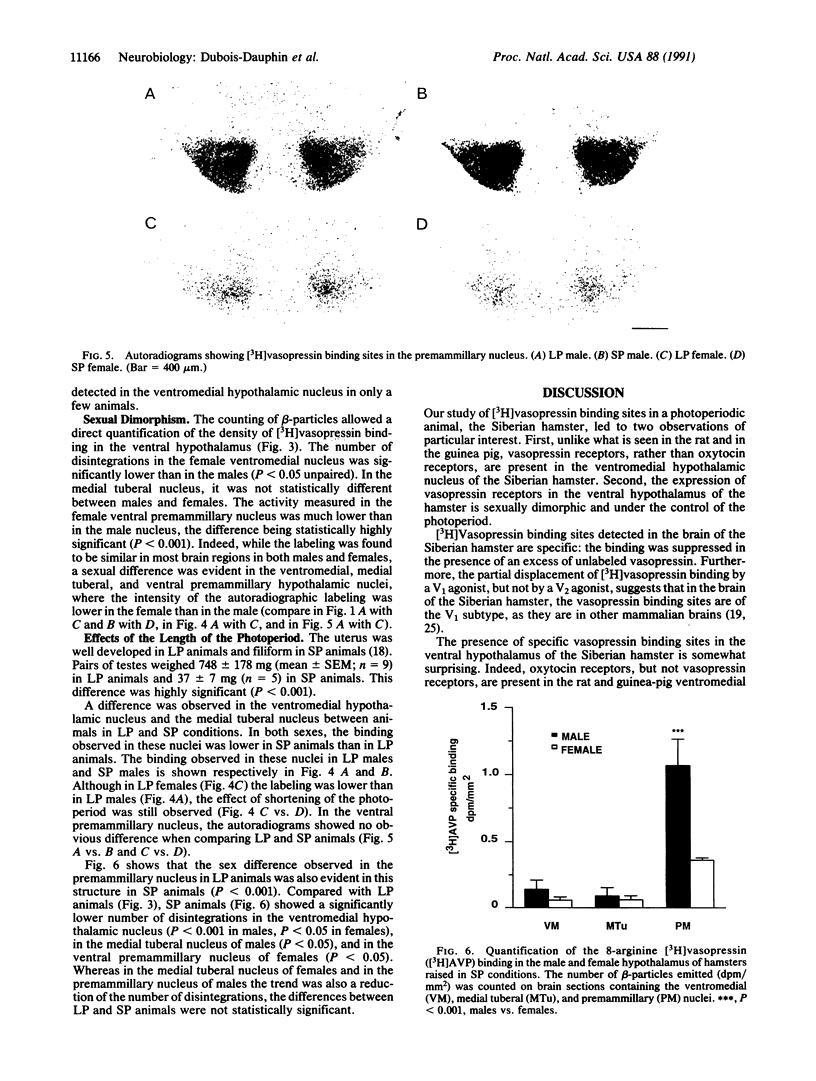
The distribution of vasopressin receptors was studied in the brain of a photoperiodic animal, the Siberian hamster. Attention was focused on [3H]vasopressin binding sites located in the hypothalamic ventromedial nucleus, medial tuberal nucleus, and ventral premammillary nucleus in males or females kept in long or short photoperiod conditions. Displacement experiments with structural analogs suggested that vasopressin receptors in the hamster hypothalamus are of the vasopressor (V1) type. Quantitative data obtained with a gaseous detector of beta-particles indicated that in the ventromedial nucleus and in the ventral premammillary nucleus of animals in long photoperiod, the number of beta-particles emitted per unit area was significantly greater in males than in females. In the ventromedial hypothalamic nucleus, in both males and females, the number of beta-particles emitted was significantly lower in short than in long photoperiod conditions. In the ventral premammillary nucleus, shortening of the photoperiod had a significant effect in reducing the amount of [3H]vasopressin bound in females, but not in males. These data suggest that, in the hamster, the control of the expression of vasopressin receptors differs among various hypothalamic nuclei and may depend on the sex and/or on the level of circulating gonadal steroids.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arletti R., Bertolini A. Oxytocin stimulates lordosis behavior in female rats. Neuropeptides. 1985 Jun;6(3):247–253. doi: 10.1016/0143-4179(85)90095-2. [DOI] [PubMed] [Google Scholar]
- Bartness T. J., Elliott J. A., Goldman B. D. Control of torpor and body weight patterns by a seasonal timer in Siberian hamsters. Am J Physiol. 1989 Jul;257(1 Pt 2):R142–R149. doi: 10.1152/ajpregu.1989.257.1.R142. [DOI] [PubMed] [Google Scholar]
- Bartness T. J., McGriff W. R., Maharaj M. P. Effects of diabetes and insulin on photoperiodic responses in Siberian hamsters. Physiol Behav. 1991 Mar;49(3):613–620. doi: 10.1016/0031-9384(91)90287-x. [DOI] [PubMed] [Google Scholar]
- Bittman E. L., Hegarty C. M., Layden M. Q., Jonassen J. A. Influences of photoperiod on sexual behaviour, neuroendocrine steroid receptors and adenohypophysial hormone secretion and gene expression in female golden hamsters. J Mol Endocrinol. 1990 Aug;5(1):15–25. doi: 10.1677/jme.0.0050015. [DOI] [PubMed] [Google Scholar]
- Buijs R. M., Pévet P., Masson-Pévet M., Pool C. W., de Vries G. J., Canguilhem B., Vivien-Roels B. Seasonal variation in vasopressin innervation in the brain of the European hamster (Cricetus cricetus). Brain Res. 1986 Apr 16;371(1):193–196. doi: 10.1016/0006-8993(86)90829-2. [DOI] [PubMed] [Google Scholar]
- Caldwell J. D., Prange A. J., Jr, Pedersen C. A. Oxytocin facilitates the sexual receptivity of estrogen-treated female rats. Neuropeptides. 1986 Feb-Mar;7(2):175–189. doi: 10.1016/0143-4179(86)90093-4. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V., Bartke A. The influence of short photoperiod on testicular and circulating levels of testosterone precursors in the adult golden hamster. Biol Reprod. 1989 Feb;40(2):300–306. doi: 10.1095/biolreprod40.2.300. [DOI] [PubMed] [Google Scholar]
- Charpak G., Dominik W., Zaganidis N. Optical imaging of the spatial distribution of beta-particles emerging from surfaces. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1741–1745. doi: 10.1073/pnas.86.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaccio L. A., Lisk R. D. Central control of estrous behavior in the female golden hamster. Estrogen sensitivity within the hypothalamus. Neuroendocrinology. 1973;13(1):21–28. doi: 10.1159/000122192. [DOI] [PubMed] [Google Scholar]
- De Vries G. J., Buijs R. M., Van Leeuwen F. W. Sex differences in vasopressin and other neurotransmitter systems in the brain. Prog Brain Res. 1984;61:185–203. doi: 10.1016/S0079-6123(08)64435-0. [DOI] [PubMed] [Google Scholar]
- DeBold J. F., Malsbury C. W., Harris V. S., Malenka R. Sexual receptivity: brain sites of estrogen action in female hamsters. Physiol Behav. 1982 Oct;29(4):589–593. doi: 10.1016/0031-9384(82)90224-4. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M., Pevet P., Tribollet E., Dreifuss J. J. Vasopressin in the brain of the golden hamster: the distribution of vasopressin binding sites and of immunoreactivity to the vasopressin-related glycopeptide. J Comp Neurol. 1990 Oct 22;300(4):535–548. doi: 10.1002/cne.903000408. [DOI] [PubMed] [Google Scholar]
- Elands J., Barberis C., Jard S., Tribollet E., Dreifuss J. J., Bankowski K., Manning M., Sawyer W. H. 125I-labelled d(CH2)5[Tyr(Me)2,Thr4,Tyr-NH2(9)]OVT: a selective oxytocin receptor ligand. Eur J Pharmacol. 1988 Mar 1;147(2):197–207. doi: 10.1016/0014-2999(88)90778-9. [DOI] [PubMed] [Google Scholar]
- Elands J., Barberis C., Jard S. [3H]-[Thr4,Gly7]OT: a highly selective ligand for central and peripheral OT receptors. Am J Physiol. 1988 Jan;254(1 Pt 1):E31–E38. doi: 10.1152/ajpendo.1988.254.1.E31. [DOI] [PubMed] [Google Scholar]
- Ferris C. F., Albers H. E., Wesolowski S. M., Goldman B. D., Luman S. E. Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden hamsters. Science. 1984 May 4;224(4648):521–523. doi: 10.1126/science.6538700. [DOI] [PubMed] [Google Scholar]
- Gorski R. A. The neuroendocrinology of reproduction: an overview. Biol Reprod. 1979 Feb;20(1):111–127. doi: 10.1093/biolreprod/20.1.111. [DOI] [PubMed] [Google Scholar]
- Hermes M. L., Buijs R. M., Masson-Pévet M., van der Woude T. P., Pévet P., Brenklé R., Kirsch R. Central vasopressin infusion prevents hibernation in the European hamster (Cricetus cricetus). Proc Natl Acad Sci U S A. 1989 Aug;86(16):6408–6411. doi: 10.1073/pnas.86.16.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K. Effects of short photoperiods on puberty, growth and moult in the Djungarian hamster (Phodopus sungorus). J Reprod Fertil. 1978 Sep;54(1):29–35. doi: 10.1530/jrf.0.0540029. [DOI] [PubMed] [Google Scholar]
- Hughes A. M., Everitt B. J., Lightman S. L., Todd K. Oxytocin in the central nervous system and sexual behaviour in male rats. Brain Res. 1987 Jun 23;414(1):133–137. doi: 10.1016/0006-8993(87)91333-3. [DOI] [PubMed] [Google Scholar]
- Johnson A. E., Ball G. F., Coirini H., Harbaugh C. R., McEwen B. S., Insel T. R. Time course of the estradiol-dependent induction of oxytocin receptor binding in the ventromedial hypothalamic nucleus of the rat. Endocrinology. 1989 Sep;125(3):1414–1419. doi: 10.1210/endo-125-3-1414. [DOI] [PubMed] [Google Scholar]
- Johnson A. E., Coirini H., Insel T. R., McEwen B. S. The regulation of oxytocin receptor binding in the ventromedial hypothalamic nucleus by testosterone and its metabolites. Endocrinology. 1991 Feb;128(2):891–896. doi: 10.1210/endo-128-2-891. [DOI] [PubMed] [Google Scholar]
- Klemcke H. G., VanSickle M., Bartke A., Amador A., Chandrashekar V. Effects of photoperiod, hypophysectomy, and follicle-stimulating hormone on testicular follicle-stimulating hormone binding sites in golden hamsters. Biol Reprod. 1987 Sep;37(2):356–370. doi: 10.1095/biolreprod37.2.356. [DOI] [PubMed] [Google Scholar]
- Krieger M. S., Morrell J. I., Pfaff D. W. Autoradiographic localization of estradiol-concentrating cells in the female hamster brain. Neuroendocrinology. 1976;22(3):193–205. doi: 10.1159/000122626. [DOI] [PubMed] [Google Scholar]
- Lisk R. D., Ferguson D. S. Neural localization of estrogen-sensitive sites for inhibition of ovulation in the golden hamster Mesocricetus auratus. Neuroendocrinology. 1973;12(3):153–160. doi: 10.1159/000122165. [DOI] [PubMed] [Google Scholar]
- MacLusky N. J., Naftolin F. Sexual differentiation of the central nervous system. Science. 1981 Mar 20;211(4488):1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Malsbury C. W., Strull D., Daood J. Half-cylinder cuts antero-lateral to the ventromedial nucleus reduce sexual receptivity in female golden hamsters. Physiol Behav. 1978 Jul;21(1):79–87. doi: 10.1016/0031-9384(78)90280-9. [DOI] [PubMed] [Google Scholar]
- Mohr E., Schmitz E. Functional characterization of estrogen and glucocorticoid responsive elements in the rat oxytocin gene. Brain Res Mol Brain Res. 1991 Mar;9(4):293–298. doi: 10.1016/0169-328x(91)90075-9. [DOI] [PubMed] [Google Scholar]
- Pfaff D., Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973 Sep 15;151(2):121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Reiter R. J. Changes in the reporductive organs of cold-exposed and light-deprived female hamsters (Mesocricetus auratus). J Reprod Fertil. 1968 Jul;16(2):217–222. doi: 10.1530/jrf.0.0160217. [DOI] [PubMed] [Google Scholar]
- SMITH O. A., Jr, BODEMER C. N. A stereotaxic atlas of the brain of the golden hamster (Mesocricetus auratus). J Comp Neurol. 1963 Feb;120:53–63. doi: 10.1002/cne.901200106. [DOI] [PubMed] [Google Scholar]
- Stoneham M. D., Everitt B. J., Hansen S., Lightman S. L., Todd K. Oxytocin and sexual behaviour in the male rat and rabbit. J Endocrinol. 1985 Oct;107(1):97–106. doi: 10.1677/joe.0.1070097. [DOI] [PubMed] [Google Scholar]
- Tribollet E., Audigier S., Dubois-Dauphin M., Dreifuss J. J. Gonadal steroids regulate oxytocin receptors but not vasopressin receptors in the brain of male and female rats. An autoradiographical study. Brain Res. 1990 Mar 12;511(1):129–140. doi: 10.1016/0006-8993(90)90232-z. [DOI] [PubMed] [Google Scholar]
- Tribollet E., Barberis C., Jard S., Dubois-Dauphin M., Dreifuss J. J. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res. 1988 Feb 23;442(1):105–118. doi: 10.1016/0006-8993(88)91437-0. [DOI] [PubMed] [Google Scholar]
- Tribollet E., Charpak S., Schmidt A., Dubois-Dauphin M., Dreifuss J. J. Appearance and transient expression of oxytocin receptors in fetal, infant, and peripubertal rat brain studied by autoradiography and electrophysiology. J Neurosci. 1989 May;9(5):1764–1773. doi: 10.1523/JNEUROSCI.09-05-01764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon S. M., Goldman B. D. Photoperiod control of reproductive development in the male Djungarian hamster (Phodopus sungorus). Endocrinology. 1984 Feb;114(2):664–670. doi: 10.1210/endo-114-2-664. [DOI] [PubMed] [Google Scholar]
- de Kloet E. R., Voorhuis D. A., Boschma Y., Elands J. Estradiol modulates density of putative 'oxytocin receptors' in discrete rat brain regions. Neuroendocrinology. 1986;44(4):415–421. doi: 10.1159/000124680. [DOI] [PubMed] [Google Scholar]