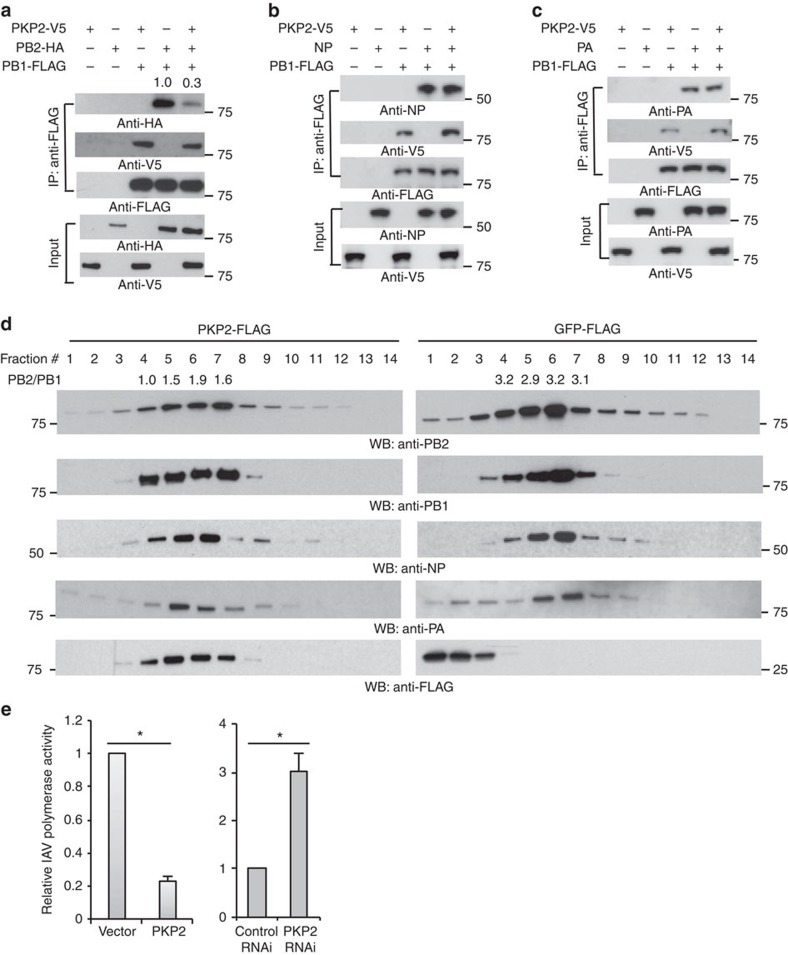
Figure 6. PKP2 perturbs the IAV polymerase complex and inhibits viral polymerase activity.
(a) PB1 and PB2 were co-transfected with PKP2 into HEK293 cells. After 48 h, cell lysates were harvested for immunoprecipitation using anti-FLAG antibody and blotted using the indicated antibodies. (b) PB1 and NP were co-transfected along with PKP2 into HEK293 cells. After 48 h, cell lysates were harvested for immunoprecipitation using anti-FLAG antibody. The indicated antibodies were used for blotting. (c). PB1 and PA were co-transfected with PKP2 into HEK293 cells. After 48 h, cell lysates were harvested for immunoprecipitation using anti-FLAG antibody and blotted as indicated. (d) HEK293 cells stably expressing PKP2-FLAG or GFP-FLAG were infected with 1 MOI of PR8 IAV for 16 h. Then the cell lysates were separated by 15–55% sucrose density centrifugation. Fractions were blotted using the indicated antibodies. The ratios of PB2 to PB1 in the fractions 4–7 were indicated. (e) HEK293 cells were transiently transfected with a plasmid cocktail containing PB1, PB2, PA, NP expression plasmids of PR8 IAV plus a polymerase I plasmid expressing an influenza virus-like RNA encoding the reporter protein firefly luciferase, along with control siRNA, PKP2 siRNA, the vector pCMV3-tag-8 or PKP2-FLAG for 48 h. The relative luciferase signal is shown. The transfection efficiency was determined by western blot (Supplementary Fig. 10b). Data represent means ±s.d. of three independent experiments. The P value was calculated (two-tailed Student's t-test) by comparison with the corresponding control. An asterisk indicates P<0.05.