Abstract
Recent studies show that Polydatin (PD) extracted from the roots of Polygonum cuspidatum Sieb, a widely used traditional Chinese remedies, possesses anti-inflammatory activity in several experimental models. In this study, we investigated the anti-inflammatory effects of PD on Staphylococcus aureus-induced mastitis in mice and elucidated the potential mechanisms. In mice with S aureus-induced mastitis, administration of PD (15, 30, 45 mg/kg, ip) or dexamethasone (Dex, 5 mg/kg, ip) significantly suppressed the infiltration of inflammatory cells, ameliorated the mammary structural damage, and inhibited the activity of myeloperoxidase, a biomarker of neutrophils accumulation. Furthermore, PD treatment dose-dependently decreased the levels of TNF-α, IL-1β, IL-6 and IL-8 in the mammary gland tissues. PD treatment also dose-dependently decreased the expression of TLR2, MyD88, IRAK1, IRAK4 and TRAF6 as well as the phosphorylation of TAK1, MKK3/6, p38 MAPK, IκB-α and NF-κB in the mammary gland tissues. In mouse mammary epithelial cells (mMECs) infected by S aureus in vitro, pretreatment with PD dose-dependently suppressed the upregulated pro-inflammatory cytokines and signaling proteins, and the nuclear translocation of NF-κB p65 and AP-1. A TLR2-neutralizing antibody mimicked PD in its suppression on S aureus-induced upregulation of MyD88, p-p38 and p-p65 levels in mMECs. PD (50, 100 μg/mL) affected neither the growth of S aureus in vitro, nor the viability of mMECs. In conclusion, PD does not exhibit antibacterial activity against S aureus, its therapeutic effects in mouse S aureus-induced mastitis depend on its ability to down-regulate pro-inflammatory cytokine levels via inhibiting TLR2-mediated activation of the p38 MAPK/NF-κB signaling pathway.
Keywords: mastitis, Staphylococcus aureus, Polydatin, dexamethasone, anti-inflammation, cytokines, TLR2, MyD88, p38 MAPK/NF-κB signaling pathway
Introduction
Mastitis, a disease that affects humans and animals worldwide, occurs most commonly in the postpartum period1,2. Mastitis is characterized by inflammation of the mammary gland tissues due to infection by etiologic microorganisms, such as the Gram-positive bacterium, Staphylococcus aureus3.
S aureus, an opportunistic bacterial pathogen, is the most prevalent pathogen in mastitis in both humans and animals4. One of the characteristics of S aureus is that it evades and influences the host immune defensive system and avoids some antibacterial agents by hiding in host cells. Thus, once mammary gland tissues are infected by S aureus, it is extremely difficult to control and eliminate the infection5.
It has been well established that recognition of the infection through activation of pattern recognition receptors (PRRs) is necessary for initiating the immune response when mammary glands are infected by S aureus6. PRRs are considered crucial in the survival of the pathogen, and they recognize pathogen-associated molecular patterns (PAMPs)7,8. TLR2, one of the PRRs, plays a key role in the innate immune response to microbial infection9, and its activation leads to the release of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IL-810. Myeloid differentiation factor 88 (MyD88) is the primary adaptor protein in the TLR2 signaling pathway and is essential for the induction of pro-inflammatory cytokines triggered by TLR211. Most of the TLRs, including TLR2, recruit MyD88 to their receptor complex, as do members of the interleukin-1 receptor family. Subsequently, MyD88 recruits interleukin-1 receptor-associated kinase 1 (IRAK1), IRAK4, and then TNF receptor-associated factor 6 (TRAF6), resulting in the activation of the NF-κB pathway, which regulates the expression of inflammatory genes12,13. In addition to the NF-κB pathway, TLR2 and IL-1Rs activate the mitogen-activated protein kinase (MAPK) pathway, in which a kinase cascade leads to the activation of c-Jun N-terminal kinase (JNK), extracellular-signal-regulated kinase (ERK) and the p38 subunit of MAPKs14. JNK/ERK/p38 MAPK phosphorylate the transcription factor AP-1, which leads to the release of pro-inflammatory cytokines15.
Polydatin (PD), extracted from the roots of Polygonum cuspidatum Sieb, is widely applied in traditional Chinese remedies16. Some studies have indicated that PD can improve heart function and ameliorate renal injury through anti-oxidative and anti-inflammatory effects17,18. However, there are no reports concerning the effects of PD on mastitis, and the mechanism involved is not clear. Accordingly, a mouse model of S aureus mastitis and mouse mammary epithelial cells (mMECs) were used in this research, and the study investigated the anti-inflammatory effects of PD on S aureus-induced mastitis and elucidated the potential mechanisms.
Materials and methods
Reagents
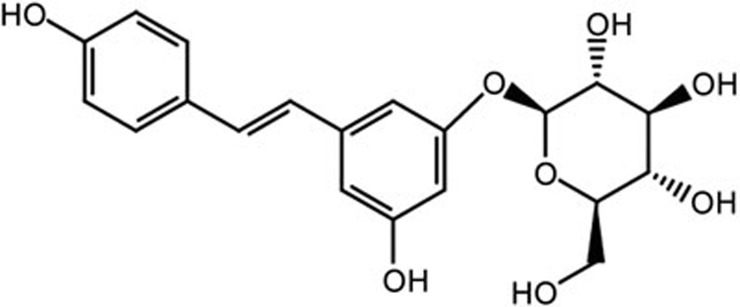
Polydatin was obtained from the National Institutes for Food and Drug Control (purity >98%, HPLC; Beijing, China) (Figure 1). Mouse TNF-α, IL-1β and IL-6 enzyme-linked immunosorbent assay (ELISA) kits were purchased from ImmunoWay Biotechnology (Newark, DE, USA). The mouse IL-8 ELISA kit was purchased from Cusabio (Wuhan, China). The nuclear extraction kit, TransAM NF-κB p65 assay kit and TransAM AP-1 ELISA kit were purchased from Active Motif (Carlsbad, CA, USA). The myeloperoxidase (MPO) determination kits were provided by the Jiancheng Bioengineering Institute of Nanjing (Nanjing, China). Primary antibodies against TLR2, MyD88, IRAK1, IRAK4, TRAF6, TAK1, p-TAK1, MKK3/6, p-MKK3/6, MAPKs, NF-κB, IκBα, p-IκBα and β-actin and the HRP-conjugated goat anti-rabbit antibody were purchased from Cell Signaling Technology (Beverly, MA, USA). All other chemical reagents were reagent grade.
Figure 1.
The chemical structure of PD.
S aureus strains and culture
S aureus strains (ATCC 25923) were purchased from the American Type Culture Collection (ATCC, MD, USA) and cultured in Luria-Bertani (LB) broth (Oxoid, Hampshire, UK) at 37 °C and 180 rounds per minute.
Animals and experimental design
Sixty adult female postpartum and lactating BALB/c mice (6–8 weeks old, weighing 35–40 g) were obtained from Wuhan Institute of Biological Products Co Ltd (Wuhan, China). All procedures were performed according to the Guide for the Care and Use of Laboratory Animals established by the US National Institutes of Health and approved by the Ethical Committee on Animal Research at Huazhong Agricultural University. The mice were housed in a room maintained at 24±1 °C with 40%–80% humidity. All animals received food and water ad libitum.
PD was dissolved in dimethyl sulfoxide (DMSO) and diluted in Dulbecco's modified Eagle's medium (DMEM). S aureus cultures were maintained in 100 mL of LB broth at 37 °C and 180 rounds per minute until they reached the stationary phase. Subsequently, the bacteria were centrifuged and then washed twice with phosphate-buffered saline (PBS, pH 7.0). The collected cell pellet was resuspended in 1 mL of PBS at 3.1×1010 CFU/mL. The method for establishing the S aureus-induced mastitis model was described previously19. Briefly, 100 μL of S aureus suspension (109 CFU) was injected into two abdominal mammary glands to induce mastitis. Then, 24 h after S aureus infection, three intraperitoneal injections of PD or DEX were given every 8 h at doses established in a previous study20. The mice were randomly divided into four groups as follows.
1. Control group (CG): The mice were treated with 100 μL of PBS as a vehicle control.
2. S aureus group (SG): The mouse model of S aureus mastitis without drug intervention.
3. PD groups: 24 h after S aureus infection, the mouse model of S aureus mastitis was intraperitoneally administered PD at 15, 30 and 45 mg/kg.
4. Dexamethasone administration group (DEX group): 24 h after S aureus infection, the mouse model of S aureus mastitis was intraperitoneally administered DEX at 5 mg/kg.
Then, 8 h after the last treatment with PD or DEX, all the mice were euthanized with sodium pentobarbital, and the mammary gland tissues were collected and stored at -80 °C for further research.
Cell culture and treatment
Primary mouse mammary epithelial cells were prepared as previously described21. Mammary gland tissues were aseptically harvested from normal pregnant BALB/c mice and minced into paste. The homogenized tissues were digested with a collagenase I/II/trypsin mixture at 37 °C and then centrifuged. The fatty layer and cell pellets were resuspended in DMEM/F12 with 10% FBS. The suspension was centrifuged again and filtered through a 40 μm pore-size filter, and the cells were then cultured at 37 °C with 5% CO2. The primary mouse mammary epithelial cells were identified using specific antibodies against cytokeratin 18.
Mouse mammary epithelial cells (mMECs) were pretreated for 1 h with PD at final concentrations of 25, 50 and 100 μg/mL. PD-untreated cells containing DEX (100 μg/mL) were used as a positive control. Subsequently, S aureus at a multiplicity of infection (MOI) of 10 was added to the cells. After 8 h of incubation at 37 °C, the cell supernatant and cells were collected for further study.
Cell viability assay
The effect of PD on mMEC viability was evaluated with an MTT assay. mMECs were incubated in the presence or absence of various concentrations of PD (25, 50 and 100 μg/mL) and DEX (100 μg/mL) for 24 h. Next, 20 μL of MTT (5 mg/mL) was added to each well and incubated for 4 h. After the supernatants were removed and the formazan was dissolved with 150 μL of DMSO in each well, the optical density (OD) value was measured at 570 nm on a microplate reader (Bio-Rad Instruments, Hercules, CA, USA).
Histopathological analysis
Mammary gland tissues were harvested and fixed with 10% formalin. Then, the tissue sections were embedded in paraffin and sectioned to 4-μm thickness. After deparaffinization and dehydration, the sections were stained with hematoxylin and eosin (H&E). The pathological changes were observed using a microscope (Olympus, Japan).
MPO activity assay
Mammary gland tissues were homogenized with reaction buffer (w/v, 1/9), and MPO activity was determined using an MPO test kit according to the manufacturer's protocols.
ELISA assay
The effects of PD on the levels of S aureus-induced pro-inflammatory cytokines were determined in mammary gland tissues and mMECs. Mammary gland tissues were homogenized and centrifuged, and the supernatant was collected. The supernatants of the mMECs with different treatments were also collected. The levels of TNF-α, IL-1 β, IL-6 and IL-8 in these supernatants were measured with ELISA kits, which were used according to the manufacturer's instructions. Finally, the OD value of each well was read at 450 nm with an automatic microplate reader (Thermo Scientific Multiskan MK3, USA).
TransAM NF-κB and AP-1 activation assays
A TransAM NF-κB p65 kit and a TransAM AP-1 Family kit were used to determine the concentrations of NF-κB p65 and AP-1 in nuclear protein extracts obtained from mMECs via a colorimetric DNA-binding ELISA, which was performed according to the manufacturer's instructions.
Quantitative real-time polymerase chain reaction (qRT-PCR) assays
Total RNA was extracted from the mammary gland tissues and mMECs using TRIzol reagent (Invitrogen, USA), according to the manufacturer's instructions. The concentration and purity of the extracted RNA were evaluated with Q5000 (Quawell Technology, USA). Total RNA (1 μg) was reverse-transcribed into first-strand DNA (cDNA) using a Prime-Script RT-PCR kit according to the manufacturer's instructions (Takara). The levels of TNF-α, IL-1β, IL-6, IL-8 and TLR2 messenger RNA (mRNA) were measured with quantitative real-time polymerase chain reaction (qRT-PCR). PCR reactions were carried out using SYBR green Plus reagent kit (Roche, Swissland) in a 25 μL reaction. Each sample was run as three replicates. The primers used in the study are listed in Table 1. The thermal cycler parameters were 95 °C for 30 s, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 60 °C for 30 s. The mRNA levels were calculated with the 2–ΔΔCt comparative method and analyzed by normalization to with GAPDH mRNA expression.
Table 1. Primers used for qPCR.
| Name | Primer sequence (5′–3′) | GenBank accession number | Product size (bp) |
|---|---|---|---|
| TLR2 | F: TTTGCTCCTGCGAACTCC | NM_011905.3 | 267 |
| R: GCCACGCCCACATCATTC | |||
| TNF-α | F: CTTCTCATTCCTGCTTGTG | NM_013693.3 | 198 |
| R: ACTTGGTGGTTTGCTACG | |||
| IL-1β | F: CCTGGGCTGTCCTGATGAGAG | NM_008361.4 | 131 |
| R: TCCACGGGAAAGACACAGGTA | |||
| IL-6 | F: GGCGGATCGGATGTTGTGAT | NM_031168.1 | 199 |
| R: GGACCCCAGACAATCGGTTG | |||
| IL-8 | F: GGTGAAGGCTACTGTTGG | NM_011339.2 | 234 |
| R: CTGGAGTCCCGTAGAAAA | |||
| GAPDH | F: CAATGTGTCCGTCGTGGATCT | NM_001289726.1 | 124 |
| R: GTCCTCAGTGTAGCCCAAGATG |
Western blot analysis
Total protein was extracted from the mMECs with RIPA reagent according to the manufacturer's recommended protocol (Biosharp, China). The total protein concentration was measured with a BCA protein assay kit (Thermo Scientific, MA, USA). Equal amounts of protein were separated with 10% SDS polyacrylamide gels, transferred onto polyvinylidene difluoride (PVDF) membranes, and blocked in 5% skim milk in TBST for 2 h. Subsequently, the membranes were incubated at 4 °C overnight with primary antibodies against TLR2, MyD88, IRAK1, IRAK4, TRAF6, TAK1, p-TAK1, MKK3/6, p-MKK3/6, MAPKs, NF-κB, IκBα, p-IκBα and β-actin (1:1000 dilutions). β-Actin was used as a control. After incubation with the secondary antibodies (1:5000 dilutions) for 2 h at 25±1 °C, protein levels were detected with an enhanced chemiluminescence reagent, and the intensities were quantified using Image J gel analysis software.
Statistical analyses
All the data in this study are presented as the mean±SEM and were analyzed with SPSS19.0 (IBM). The differences between groups were assessed using one-way ANOVA with the LSD multiple comparison test and Student's t-test. Statistical significance was defined as P<0.05.
Results
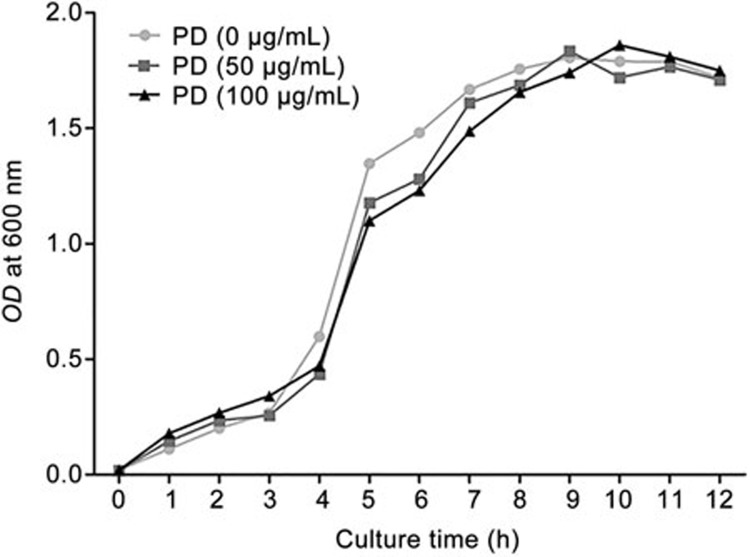
Effects of PD on S aureus growth
To investigate whether PD had antibacterial activity against S aureus, the growth curve of S aureus at different concentrations of PD was determined. Our results suggested that PD had little effect on S aureus growth (Figure 2).
Figure 2.
The effects of PD on S aureus growth. S aureus was cultured in LB with various concentrations of PD (0, 50, 100 μg/mL). The OD value was measured every 1 h.
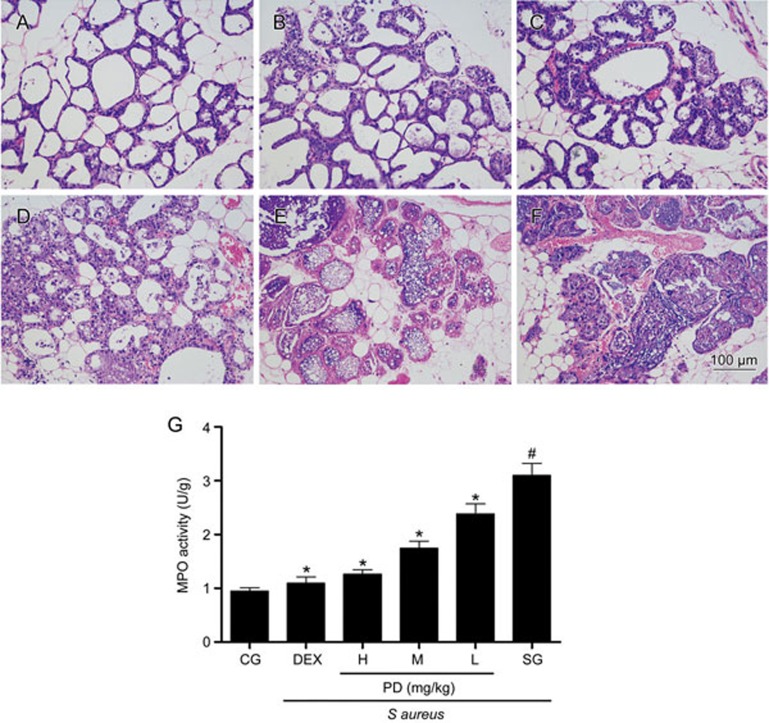
Effects of PD on S aureus-induced histopathological changes
There were no histopathological changes in the control group (Figure 3A). In the S aureus group, the lobules of the mammary gland were not complete, and a large number of inflammatory cells were observed in the mammary gland tissues. Moreover, the acini of the mammary glands were also damaged (Figure 3F). However, these histopathological changes were alleviated in the PD groups and DEX group, and the lobules were complete and the number of inflammatory cells decreased (Figure 3B–3E).
Figure 3.
The effects of PD on S aureus-induced mammary gland injury. Mammary gland tissues were harvested 8 h after the last treatment with PD or DEX. (A) Control group. (B) Dexamethasone group. (C–E) PD (45, 30, 15 mg/kg) groups and (F) S aureus group. (G) MPO activity assay. CG is the control group; SG is the S aureus group; DEX is the dexamethasone group; and H, M and L are the PD administration groups, with doses of 45, 30 and 15 mg/kg, respectively. The data are presented as the mean±SEM. *P<0.05 vs SG. #P<0.05 vs CG.
Effects of PD on MPO activity
As an important indicator of neutrophil accumulation in the mammary gland tissues, MPO activity was measured in the present study. The results are shown in Figure 3G. We observed that infection with S aureus dramatically increased MPO activity in mammary gland tissues relative to the control group. However, these increases were suppressed by PD in a dose-dependent manner.
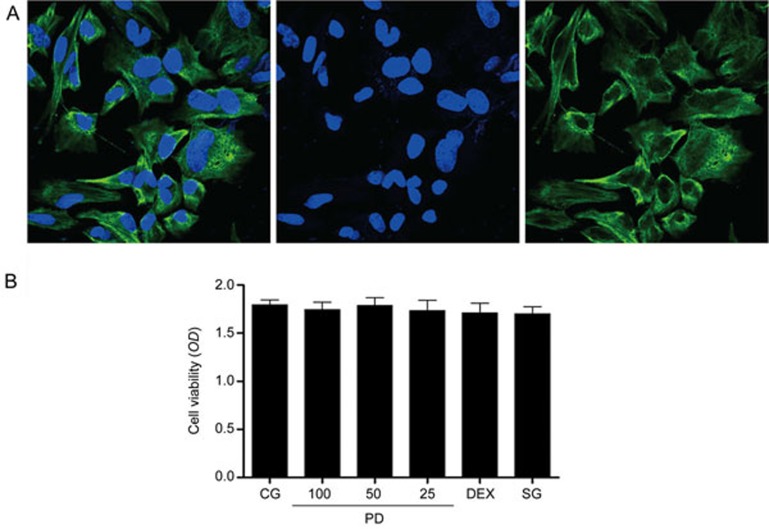
Cell integrity and cell viability assay
Cytokeratin 18 is widely used to identify the integrity of mammary gland epithelial cells19. In our study, mMECs were pretreated with a blue fluorophore to observe the cell nucleus, and cytokeratin 18 was labeled with a green fluorophore to identify cell integrity (Figure 4A). The cytotoxicity of PD to mMECs was assessed with an MTT assay. The results showed that cell viability was not affected by PD treatment (25, 50 and 100 μg/mL) (Figure 4B).
Figure 4.
Mouse mammary epithelial cell identification and cell viability. (A) Mouse mammary epithelial cells (mMECs) were pretreated with a fluorophore to observe their integrity. Cytokeratin 18 was labeled with a green fluorophore, and the cell nucleus was labeled by a blue fluorophore. (B) The effects of PD on the viability of mMECs. The cells were cultured with different concentrations of PD (25, 50 and 100 μg/mL). Cell viability was determined with an MTT assay. The data are presented as the mean±SEM.
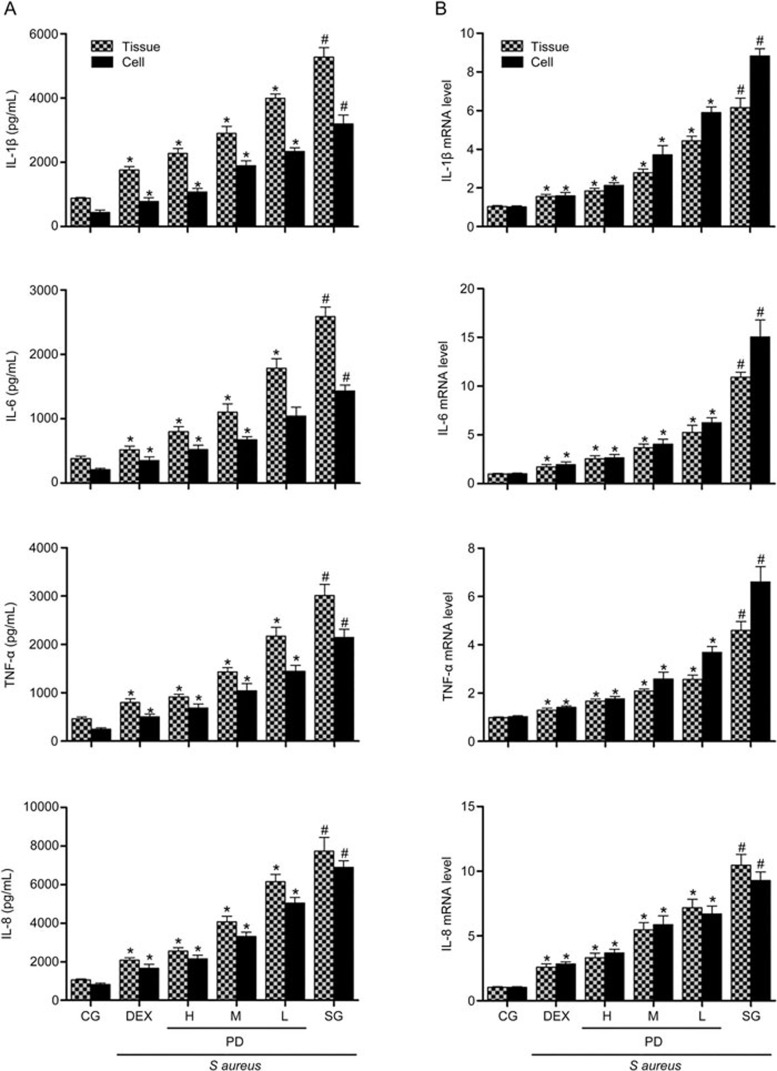
Effects of PD on inflammatory cytokines
Many studies have shown that the production of pro-inflammatory cytokines plays an essential role in inflammatory responses; thus, we investigated whether PD could inhibit the secretion of TNF-α, IL-1β, IL-6 and IL-8 in S aureus-induced mastitis. The results are shown in Figures 5A and 5B. The TNF-α, IL-1β, IL-6 and IL-8 levels in the S aureus group were significantly increased relative to the control group. However, these increases were significantly inhibited by PD and DEX. With increasing doses of PD, the effect was more pronounced. Additional studies performed on mMECs produced the same results as those observed in the mammary gland tissues (Figures 5A and 5B).
Figure 5.
The effects of PD on the production of pro-inflammatory cytokines. (A) The levels of the cytokines TNF-α, IL-1β, IL-6 and IL-8 induced by S aureus were determined with an ELISA. (B) The S aureus-induced expression of the cytokines TNF-α, IL-1β, IL-6 and IL-8 was determined with qPCR. GAPDH was used as a control. CG is the control group; SG is the S aureus group; DEX is the dexamethasone group; and H, M and L are the PD administration groups, with doses of 45, 30 and 15 mg/kg per animal, respectively, and 100, 50 and 25 μg/mL in the cells, respectively. The data are presented as the mean±SEM. *P<0.05 vs SG. #P<0.05 vs CG.
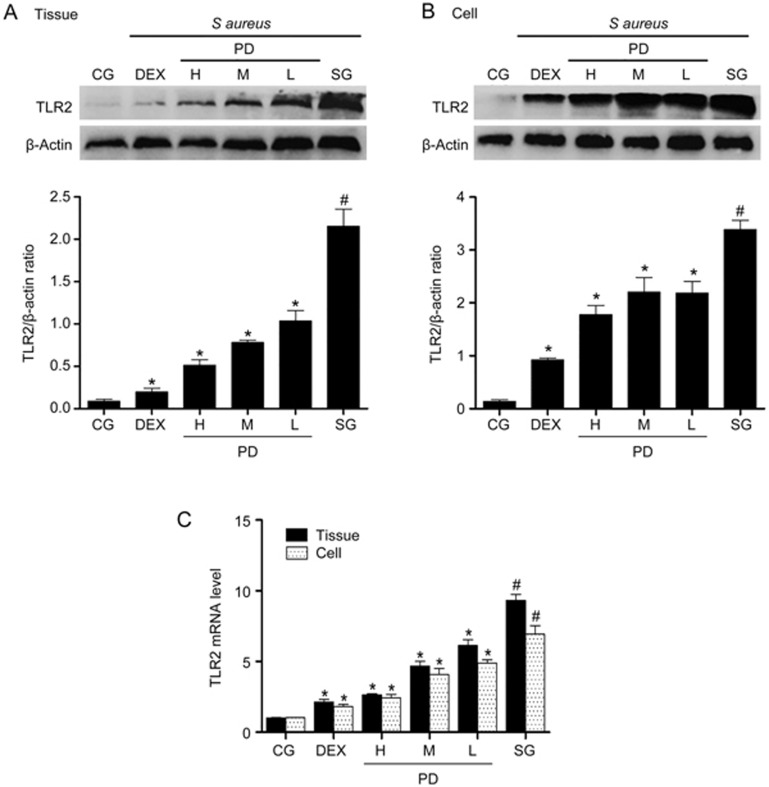
Effects of PD on TLR2 expression
TLR2 plays a pivotal role in the S aureus-induced inflammatory response22. To explore whether PD could affect TLR2, the expression of TLR2 was measured with qPCR and Western blotting. The expression of TLR2 was significantly higher in the S aureus group than in the control group. However, PD significantly inhibited these increases in a dose-dependent manner (Figure 6).
Figure 6.
The effect of PD on TLR2 expression. (A) The protein expression of TLR2 in tissues was measured with Western blotting. (B) The protein expression of TLR2 in mMECs. β-Actin was used as a control. (C) The mRNA expression of TLR2 in tissues and mMECs was determined with qPCR. GAPDH was used as a control. CG is the control group; SG is the S aureus group; DEX is the dexamethasone group; and H, M and L are the PD administration groups, with doses of 45, 30 and 15 mg/kg per animal, respectively, and 100, 50 and 25 μg/mL in the cells, respectively. The data are presented as the mean±SEM. *P<0.05 vs SG. #P<0.05 vs CG.
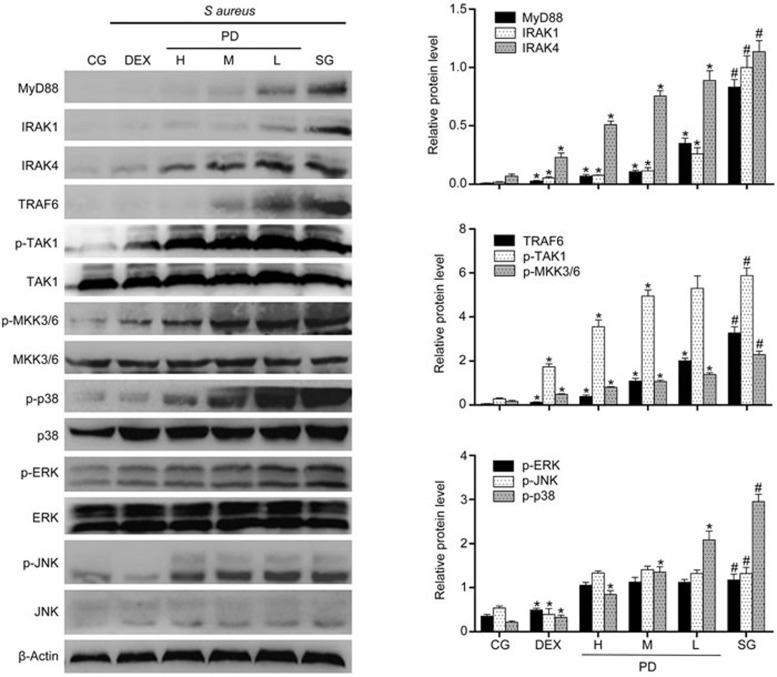
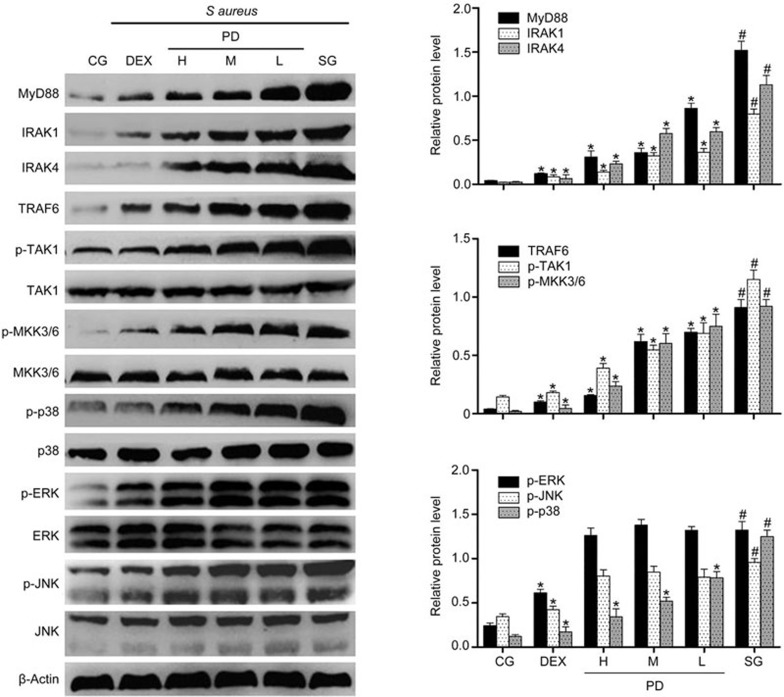
Effects of PD on the MyD88-MAPK signaling pathway
To further investigate the anti-inflammatory mechanism of PD, Western blotting was performed to measure the expression of proteins in the MyD88-MAPK signaling pathway in tissues. Compared with the control group, the S aureus group exhibited significantly increased MyD88, IRAK1, IRAK4, TRAF6, p-TAK1, p-MKK3/6 and MAPK expression. However, PD significantly blocked the increase in MyD88, IRAK1, IRAK4 and TRAF6 levels and the phosphorylation of TAK1, MKK3/6 and p38 but did not influence the level of p-JNK or p-ERK (Figure 7). To further confirm the results, the same study was performed using mMECs. The results were consistent with those obtained using the tissues (Figure 8).
Figure 7.
The effects of PD on the TLR2-mediated activation of the MAPK signaling pathway in tissues. The expression of MyD88, IRAK1, IRAK4, TRAF6, TAK1, MKK3/6 and MAPK protein in tissues was determined using a Western blot. β-Actin was used as a control. CG is the control group; SG is the S aureus group; DEX is the dexamethasone group; and H, M and L are the PD administration groups, with doses of 45, 30 and 15 mg/kg per animal, respectively, and 100, 50 and 25 μg/mL in the cells, respectively. The data are presented as the mean±SEM. *P<0.05 vs SG. #P<0.05 vs CG.
Figure 8.
The effects of PD on the TLR2-mediated activation of the MAPK signaling pathway in mMECs. The expression of MyD88, IRAK1, IRAK4, TRAF6, TAK1, MKK3/6 and MAPK protein in mMECs was determined using Western blotting. β-Actin was used as a control. CG is the control group; SG is the S aureus group; DEX is the dexamethasone group; and H, M and L are the PD administration groups, with doses of 45, 30 and 15 mg/kg per animal, respectively, and 100, 50 and 25 μg/mL in the cells, respectively. The data are presented as the mean±SEM. *P<0.05 vs SG. #P<0.05 vs CG.
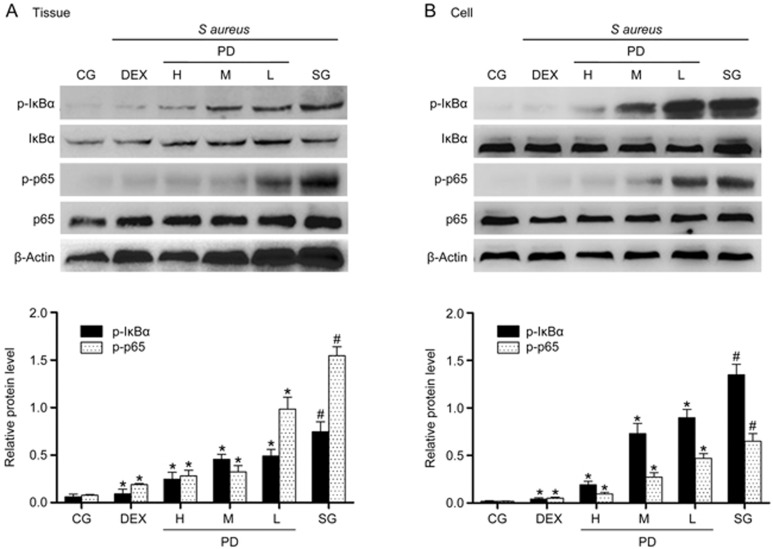
Effects of PD on the NF-κB signaling pathway
NF-κB is a crucial mediator in the inflammatory response and can also be activated by TLR223. The levels of p-IκBα and p-p65 were significantly increased in the S aureus group relative to the control group. In contrast, their levels were greatly decreased in the PD treatment group (Figure 9).
Figure 9.
The effects of PD on the NF-κB signaling pathway. (A) The expression of IκBα and p65 protein in tissues. (B) The expression of IκBα and p65 protein in mMECs. β-Actin was used as a control. CG is the control group; SG is the S aureus group; DEX is the dexamethasone group; and H, M and L are the PD administration groups, with doses of 45, 30 and 15 mg/kg per animal, respectively, and 100, 50 and 25 μg/mL in the cells, respectively. The data are presented as the mean±SEM. *P<0.05 vs SG. #P<0.05 vs CG.
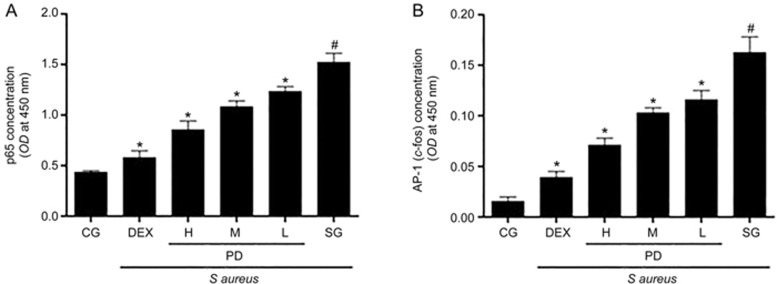
Effects of PD on the nuclear translocation of NF-κB p65 and AP-1
To study the effects of PD on the nuclear translocation of NF-κB p65 and AP-1, we used TransAM assays to measure the concentrations of NF-κB p65 and AP-1 in nuclear protein extracts, as described previously24. The nuclear translocation of both NF-κB p65 and AP-1 was markedly enhanced after inoculation with S aureus in the S aureus group. However, pretreatment with PD significantly attenuated these increases (Figure 10).
Figure 10.
The effects of PD on the nuclear translocation of NF-κB p65 and AP-1. The mMECs were pretreated with or without PD for 1 h and were subjected to S aureus for an additional 8 h. The concentrations of NF-κB p65 and AP-1 in nuclear protein extracts from the cells were measured using TransAM assays. (A) The concentration of NF-κB p65 in nuclear protein extracts from mMECs. (B) The concentration of AP-1 in nuclear protein extracts from mMECs. CG is the control group; SG is the S aureus group; DEX is the dexamethasone group; and H, M and L are the PD administration groups, with doses of 100, 50 and 25 μg/mL, respectively. The data are presented as the mean±SEM. *P<0.05 vs SG. #P<0.05 vs CG.
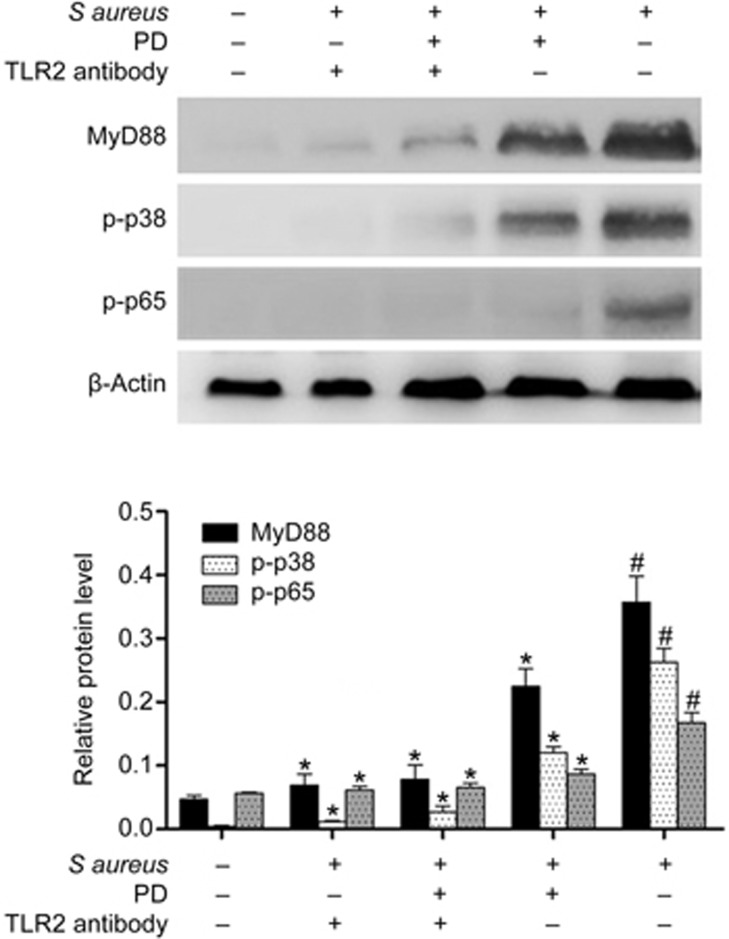
Effects of PD were mediated through TLR2
Additionally, to confirm whether the anti-inflammatory effects of PD were mediated through TLR2, mMECs were pretreated with a TLR2-neutralizing antibody (1 μg/mL, InvivoGen) or PD (100 μg/mL) for 1 h and then incubated with S aureus for 8 h. The protein levels of MyD88, p-p38 and p-p65 were determined by Western blotting. The results showed that the TLR2-neutralizing antibody significantly decreased S aureus-induced MyD88, p-p38 and p-p65 levels, and a similar effect was observed for PD, indicating that the anti-inflammatory effects of PD were mediated through TLR2 (Figure 11).
Figure 11.
The effects of PD were mediated through TLR2. The mMECs were pretreated with a TLR2-neutralizing antibody (1 μg/mL) or PD (100 μg/mL) for 1 h and then incubated with S aureus for 8 h. The levels of MyD88, p-p38 and p-p65 were determined by Western blotting. The data are presented as the mean±SEM. *P<0.05 vs SG. #P<0.05 vs CG.
Discussion
Polydatin (PD), which is extracted from the roots of Polygonum cuspidatum Sieb, has been widely used in the clinical treatment of various diseases, including LPS-stimulated ALI in rats20. In addition, it has also been reported to exert anti-inflammatory effects in RAW 264.7 cells25. However, few reports have focused on the effects of PD in S aureus-induced mastitis. In the present study, we investigated the anti-inflammatory effects of PD on S aureus-induced mastitis in vivo and in vitro.
S aureus, a Gram-positive bacterium, is one of the major pathogens that causes mastitis4. The intramammary instillation of S aureus is a well-known model of mastitis in mice19. In our study, it was observed in the histopathological analysis that PD clearly suppressed the infiltration of inflammatory cells and reversed the mammary structural damage. Moreover, MPO is a biomarker of neutrophil influx into tissues and reflects the number of neutrophils in mammary gland tissues26. The MPO assay results showed that PD significantly inhibited MPO activity in S aureus-induced mastitis. Moreover, an antibacterial test was also performed to explore whether PD has antibacterial activity against S aureus. The results showed that PD did not exert its anti-inflammatory effects by inhibiting bacterial proliferation, indicating that an additional anti-inflammatory mechanism exists.
S aureus infection leads to the overwhelming release of the pro-inflammatory cytokines TNF-α, IL-1β, IL-6, and IL-8, which play pivotal roles in the initiation and regulation of inflammatory responses27. These pro-inflammatory cytokines also participate in the recruitment of neutrophils and the promotion of MPO activity. In this study, the TNF-α, IL-1β, IL-6 and IL-8 levels in tissues and mMECs were significantly lower in the PD group than in the S aureus group. The results were also in accordance with the MPO results. Moreover, PD at 45 mg/kg resulted in anti-inflammatory effects similar to those of dexamethasone. These results revealed that PD exerted anti-inflammatory effects by regulating pro-inflammatory cytokine levels in S aureus-induced mastitis.
TLR2, a key immune receptor in the innate immune system, was found to be activated by the S aureus cell wall components peptidoglycan and lipoteichoic acid, and TLR2 expression increased significantly after infection28. Recent studies have also shown that TLR2 plays a very important role in inflammatory responses by regulating MyD88, leading to the activation of MAPKs and NF-κB and the subsequent production of pro-inflammatory cytokines29. MyD88 is a signaling adaptor protein that is critical in the immune response against a wide range of microbial pathogens30 and is necessary for the phosphorylation and activation of IRAK1 through the recruitment of IRAK431. Activated IRAK is released from the receptor complex and interacts with TRAF6 and TAB2 (TAK1 binding protein 2), which mediates the translocation of TRAF6 and TAB2 to the cytosol and ultimately results in the activation of TAK132,33. Subsequently, activated TAK1 phosphorylates MAPK kinase 3/6 (MKK3/6), leading to the activation of MAPKs and NF-κB33,34. MAPKs have been reported to play a central role in S aureus-induced inflammation and can also activate AP-1, which acts similarly to NF-κB p65 to promote the expression of pro-inflammatory cytokines35. To further understand the molecular mechanism through which PD exerts its anti-inflammatory effects, we explored the TLR2-mediated activation of the MAPK/NF-κB signaling pathway. Our results suggested that PD significantly inhibited the expression of TLR2, MyD88, IRAK1, IRAK4, TRAF6, p-TAK1 and p-MKK3/6. Consequently, pretreatment with PD significantly suppressed the activation of p38 MAPK, AP-1, IκB-α and NF-κB but did not influence the level of p-JNK or p-ERK, which is consistent with previous research36. Additionally, we further confirmed the role of TLR2 in S aureus-induced mastitis using a TLR2-neutralizing antibody; the results were identical to those for PD and indicated that the anti-inflammatory effects of PD were mediated through TLR2.
In conclusion, the results demonstrated that PD exerted anti-inflammatory effects in S aureus-induced mastitis by inhibiting the TLR2-mediated activation of the p38 MAPK/NF-κB signaling pathway. The findings of this study indirectly suggest that PD may be an effective drug in the treatment of S aureus-induced mastitis.
Author contribution
Kang-feng JIANG, Gan ZHAO, and Gan-zhen DENG conceived and designed the experiments; Kang-feng JIANG, Hai-chong WU, and Xiu-ying CHEN performed the experiments; Kang-feng JIANG and Nan-nan YIN analyzed the data; and Xiu-li PENG helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No 31272631 and 31472254).
References
- Fernández L, Arroyo R, Espinosa I, Marín M, Jiménez E, Rodríguez J. Probiotics for human lactational mastitis. Benef Microbes 2014; 5: 169–83. [DOI] [PubMed] [Google Scholar]
- Miao J, Zhang J, Ma Z, Zheng L. The role of NADPH oxidase in taurine attenuation of Streptococcus uberis-induced mastitis in rats. Int Immunopharmacol 2013; 16: 429–35. [DOI] [PubMed] [Google Scholar]
- Sears PM, McCarthy KK. Management and treatment of staphylococcal mastitis. Vet Clin North Am Food Anim Pract 2003; 19: 171–85. [DOI] [PubMed] [Google Scholar]
- Niebyl JR, Spence M, Parmley T. Sporadic (nonepidemic) puerperal mastitis. J Reprod Med 1978; 20: 97–100. [PubMed] [Google Scholar]
- Twomey DP, Wheelock AI, Flynn J, Meaney WJ, Hill C, Ross RP. Protection against Staphylococcus aureus mastitis in dairy cows using a bismuth-based teat seal containing the bacteriocin, lacticin 3147. J Dairy Sci 2000; 83: 1981–8. [DOI] [PubMed] [Google Scholar]
- Elazar S, Gonen E, Livneh-Kol A, Rosenshine I, Shpigel NY. Neutrophil recruitment in endotoxin-induced murine mastitis is strictly dependent on mammary alveolar macrophages. Vet Res 2010; 41: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler BA. TLRs and innate immunity. Blood 2009; 113: 1399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature 2007; 449: 819–26. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. TLR signaling pathways. Semin Immunol 2004; 16: 3–9. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140: 805–20. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature 2002; 420: 324–9. [DOI] [PubMed] [Google Scholar]
- O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev 2008; 226: 10–8. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006; 124: 783–801. [DOI] [PubMed] [Google Scholar]
- Janssens S, Beyaert R. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem Sci 2002; 27: 474–82. [DOI] [PubMed] [Google Scholar]
- Carneiro DMVF, Domingues PF, Vaz AK. Innate immunity of the bovine mammary gland: response to infection. Ciência Rural 2009; 39: 1934–43. [Google Scholar]
- Xing WW, Wu JZ, Jia M, Du J, Zhang H, Qin LP. Effects of polydatin from polygonum cuspidatum on lipid profile in hyperlipidemic rabbits. Biomed Pharmacother 2009; 63: 457–62. [DOI] [PubMed] [Google Scholar]
- Chen L, Lan Z, Lin Q, Mi X, He Y, Wei L, et al. Polydatin ameliorates renal injury by attenuating oxidative stress-related inflammatory responses in fructose-induced urate nephropathic mice. Food Chem Toxicol 2013; 52: 28–35. [DOI] [PubMed] [Google Scholar]
- Jiang X, Liu W, Deng J, Lan L, Xue X, Zhang C, et al. Polydatin protects cardiac function against burn injury by inhibiting sarcoplasmic reticulum Ca2+ leak by reducing oxidative modification of ryanodine receptors. Free Radic Biol Med 2013; 60: 292–9. [DOI] [PubMed] [Google Scholar]
- Guo MY, Li WY, Zhang Z, Qiu C, Li C, Deng G. Betulin suppresses S aureus-induced mammary gland inflammatory injury by regulating PPAR-γ in mice. Int Immunopharmacol 2015; 29: 824–31. [DOI] [PubMed] [Google Scholar]
- Li T, Liu Y, Li G, Wang X, Zeng Z, Cai S, et al. Polydatin attenuates ipopolysaccharide-induced acute lung injury in rats. Int J Clin Exp Pathol 2014; 7: 8401. [PMC free article] [PubMed] [Google Scholar]
- Hu X, Fu Y, Tian Y, Zhang Z, Zhang W, Gao X, et al. The anti-inflammatory effect of TR6 on LPS-induced mastitis in mice. Int Immunopharmacol 2016; 30: 150–6. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 2000; 165: 5392–6. [DOI] [PubMed] [Google Scholar]
- Sheu F, Chien PJ, Hsieh KY, Chin KL, Huang WT, Tsao CY, et al. Purification, cloning, and functional characterization of a novel immunomodulatory protein from Antrodia camphorata (bitter mushroom) that exhibits TLR2-dependent NF-κB activation and M1 polarization within murine macrophages. J Agric Food Chem 2009; 57: 4130–41. [DOI] [PubMed] [Google Scholar]
- Ravichandran P, Baluchamy S, Sadanandan B, Gopikrishnan R, Biradar S, Ramesh V, et al. Multiwalled carbon nanotubes activate NF-kappaB and AP-1 signaling pathways to induce apoptosis in rat lung epithelial cells. Apoptosis 2010; 15: 1507–16. [DOI] [PubMed] [Google Scholar]
- Lou T, Jiang W, Xu D, Chen T, Fu Y. Inhibitory effects of polydatin on lipopolysaccharide-stimulated RAW 264.7 cells. Inflammation 2015; 38: 1213–20. [DOI] [PubMed] [Google Scholar]
- Li HM, Wang YY, Wang HD, Cao WJ, Yu XH, Lu DX, et al. Berberine protects against lipopolysaccharide-induced intestinal injury in mice via alpha 2 adrenoceptor-independent mechanisms. Acta Pharmacol Sin 2011; 32: 1364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL-1 and TNF). FASEB J 1990; 4: 2860–7. [PubMed] [Google Scholar]
- Yang W, Zerbe H, Petzl W, Brunner RM, Günther J, Draing C, et al. Bovine TLR2 and TLR4 properly transduce signals from Staphylococcus aureus and E coli, but S aureus fails to both activate NF-κB in mammary epithelial cells and to quickly induce TNFα and interleukin-8 (CXCL8) expression in the udder. Mol Immunol 2008; 45: 1385–97. [DOI] [PubMed] [Google Scholar]
- Basset A, Zhang F, Benes C, Sayeed S, Herd M, Thompson C, et al. Toll-like receptor (TLR) 2 mediates inflammatory responses to oligomerized RrgA pneumococcal pilus type 1 protein. J Biol Chem 2013; 288: 2665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 2004; 4: 499–511. [DOI] [PubMed] [Google Scholar]
- Janssens S, Burns K, Tschopp J, Beyaert R. Regulation of interleukin-1 and lipopolysaccharide-induced NF-κB activation by alternative splicing of MyD88. Curr Biol 2002; 12: 467–71. [DOI] [PubMed] [Google Scholar]
- Qian Y, Commane M, Ninomiya-Tsuji J, Matsumoto K, Li X. IRAK-mediated translocation of TRAF6 and TAB2 in the interleukin-1-induced activation of NFκB. J Biol Chem 2001; 276: 41661–7. [DOI] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J-i, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 2001; 412: 346–51. [DOI] [PubMed] [Google Scholar]
- Pang HY, Liu G, Liu GT. Compound FLZ inhibits lipopolysaccharide-induced inflammatory effects via down-regulation of the TAK-IKK and TAK-JNK/p38MAPK pathways in RAW264.7 macrophages. Acta Pharmacol Sin 2009; 30: 209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Song X, Zhang Z, Guo M, Jiang H, Wang W, et al. Stevioside inhibits inflammation and apoptosis by regulating TLR2 and TLR2-related proteins in S aureus-infected mouse mammary epithelial cells. Int Immunopharmacol 2014; 22: 192–9. [DOI] [PubMed] [Google Scholar]
- Alva-Murillo N, Medina-Estrada I, Báez-Magaña M, Ochoa-Zarzosa A, López-Meza JE. The activation of the TLR2/p38 pathway by sodium butyrate in bovine mammary epithelial cells is involved in the reduction of Staphylococcus aureus internalization. Mol Immunol 2015; 68: 445–55. [DOI] [PubMed] [Google Scholar]