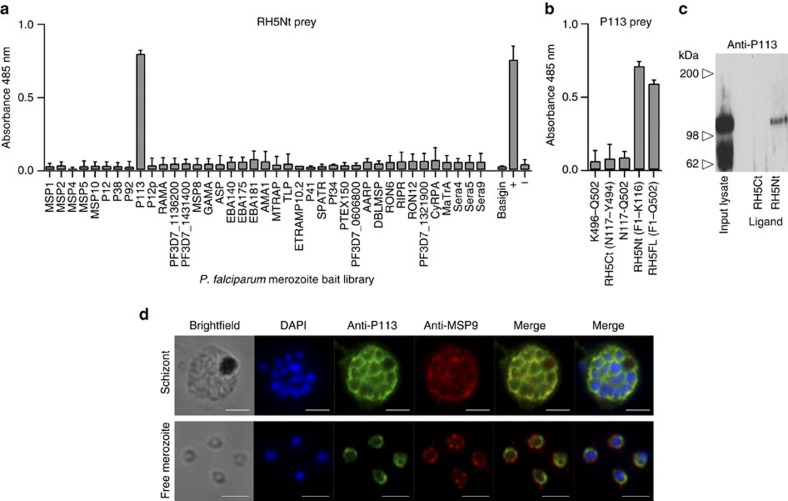
Figure 2. The N-terminal domain of RH5 interacts with the merozoite surface protein P113.
(a) The N-terminal domain of RH5 (RH5Nt, F1-K116) was expressed as a pentameric, β-lactamase-tagged prey protein and systematically tested for interactions against a library of recombinant P. falciparum merozoite receptor ectodomains and secreted bait proteins using the AVEXIS assay; a single interaction with P113 was observed. (b) The P113-RH5Nt interaction detected in the reciprocal orientation using a P113 prey against RH5 baits. Bars in (a,b) represent means±95% confidence intervals; n=3; controls were RH5FL-basigin (+ve) and Cd4d3+4 tag alone (−ve). (c) Biochemical purification of native P113 from P. falciparum cultures with RH5Nt but not control RH5Ct-coated agarose beads. Parasite lysates were incubated with beads coated in either RH5Nt or RH5Ct protein, washed, and eluates resolved by SDS–PAGE under reducing conditions, blotted and probed with anti-P113 antibody. (d) P113 is expressed in schizonts and on the surface of free merozoites. Fixed blood-stage P. falciparum schizonts and free merozoites were probed with anti-P113 antibodies (green), the merozoite surface marker anti-MSP9 (red) and nucleic acid stained with DAPI (blue). Scale bars, 3 μm.