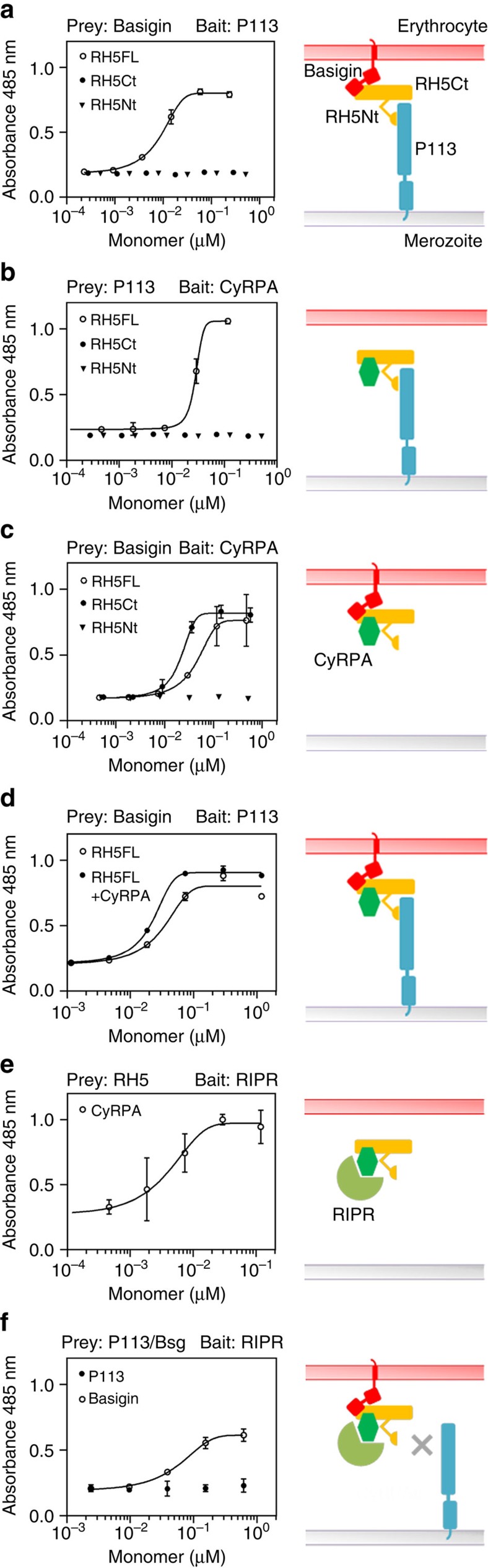
Figure 5. The RH5-CyRPA-RIPR complex can interact with basigin but not P113.
The binding interdependencies of the proteins within the RH5 invasion complex with basigin and P113 were determined using modified AVEXIS assays. The indicated purified monomeric components of the RH5 complex were titrated into binding reactions between the named baits and β-lactamase-tagged preys, which do not interact directly, and any resulting prey binding quantified by measuring the hydrolysis of a colorimetric β-lactamase substrate at 485 nm. Binding data are shown on the left panels with their interpretations shown schematically on the right. RH5FL can simultaneously bind P113 and basigin (a), CyRPA and P113 (b), and CyRPA and basigin (c). (d) The basigin-RH5FL-P113 complex is not overtly affected by the addition of CyRPA; here, the RH5FL monomer concentration was titrated with CyRPA held constant at 0.3 μM. (e) RH5FL prey could be captured on a RIPR bait by addition of purified CyRPA. (f) The RH5FL-CyRPA-RIPR complex interacted with basigin, but not P113 preys; here, the CyRPA monomer concentration was titrated with RH5FL held constant at 0.2 μM. Binding data points represent means±95% confidence interval (n=3); a representative experiment from at least two independent experiments is shown.