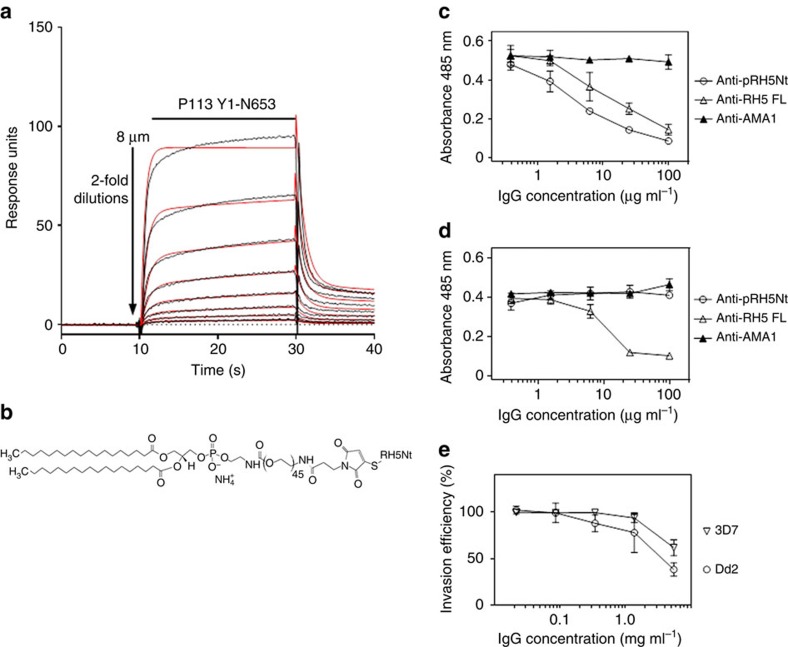
Figure 6. An ‘amph-vaccine' based on RH5Nt elicits antibodies that inhibit parasite growth in vitro.
(a) A synthetic 116 amino-acid peptide corresponding to RH5Nt interacts with P113. Serial dilutions of purified P113 (Y1-N653) were injected over the RH5Nt peptide immobilized on a streptavidin-coated sensor chip after the C-terminal cysteine was conjugated to biotin functionalized with maleimide. Reference-subtracted binding data are shown (black lines) which fit well to a 1:1 binding model (red lines). (b) Structure of an ‘amph-vaccine' based on RH5Nt created by conjugating the RH5Nt peptide to maleimide-functionalized 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (PEG2000-DSPE). Antibodies raised against RH5Nt peptide (Anti-pRH5Nt) blocked RH5 binding to P113 (c), but not to basigin (d). The indicated concentrations of protein-G purified rabbit polyclonal antibodies were incubated with RH5 β-lactamase-tagged prey proteins before presenting them to immobilized P113 (c) or basigin (d) baits. Prey binding was quantified by nitrocefin hydrolysis at 485 nm; polyclonal antibodies to RH5FL and AMA1 were used as positive and negative controls respectively. (e) Polyclonal antibodies elicited against the RH5Nt amph-vaccine inhibited erythrocyte invasion of both 3D7 and Dd2 strain of P. falciparum. Data points represent means±95% confidence interval, n=3; a representative experiment from two independent experiments is shown.