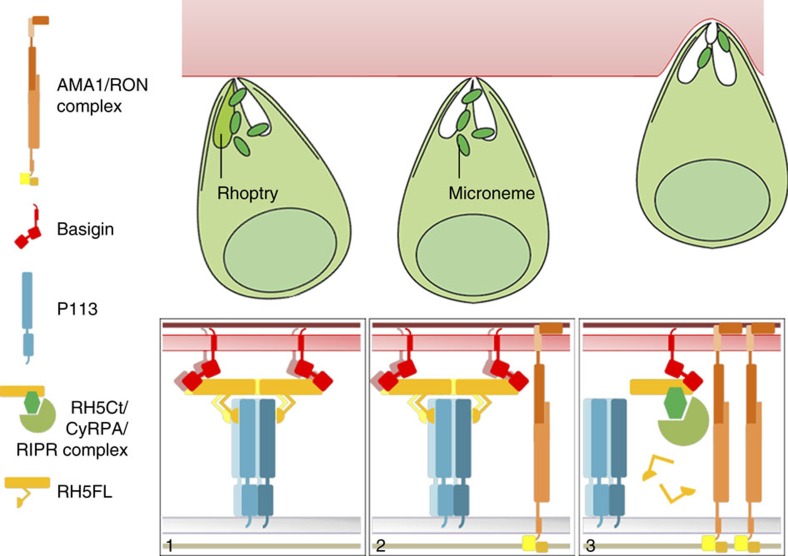
Figure 7. A model describing the role of the RH5 complex and its interaction with P113 during erythrocyte invasion.
Before invasion, the different components of the RH5 invasion complex are segregated within different subcellular locations of the merozoite and therefore purposefully prevented from interacting: RH5 is located in the rhoptries, CyRPA and RIPR in the micronemes, and P113 on the surface of the merozoite. Following engagement of the erythrocyte and release of the rhoptry contents, RH5 is tethered via its N-terminal region at the merozoite membrane by the surface-localized multimeric P113, enabling direct presentation to the basigin receptor on the erythrocyte surface and leading to the formation of an open connection for other invasion ligands to be secreted (1). The AMA1/RON complex initiates the formation of the moving junction (2). The localized secretion of CyRPA and RIPR from the micronemes leads to the formation of the RH5-CyRPA-RIPR-basigin invasion complex which, either because of the cleavage of RH5 to remove the N-terminal region, and/or because P113 and RIPR cannot simultaneously bind the RH5 complex would release RH5 from the P113 tether at the merozoite surface to licence invasion (3). The P113-RH5 complex would therefore only be fleetingly formed during the rapid invasion process resulting in a soluble post-invasion RH5Ct-CyRPA-RIPR complex.