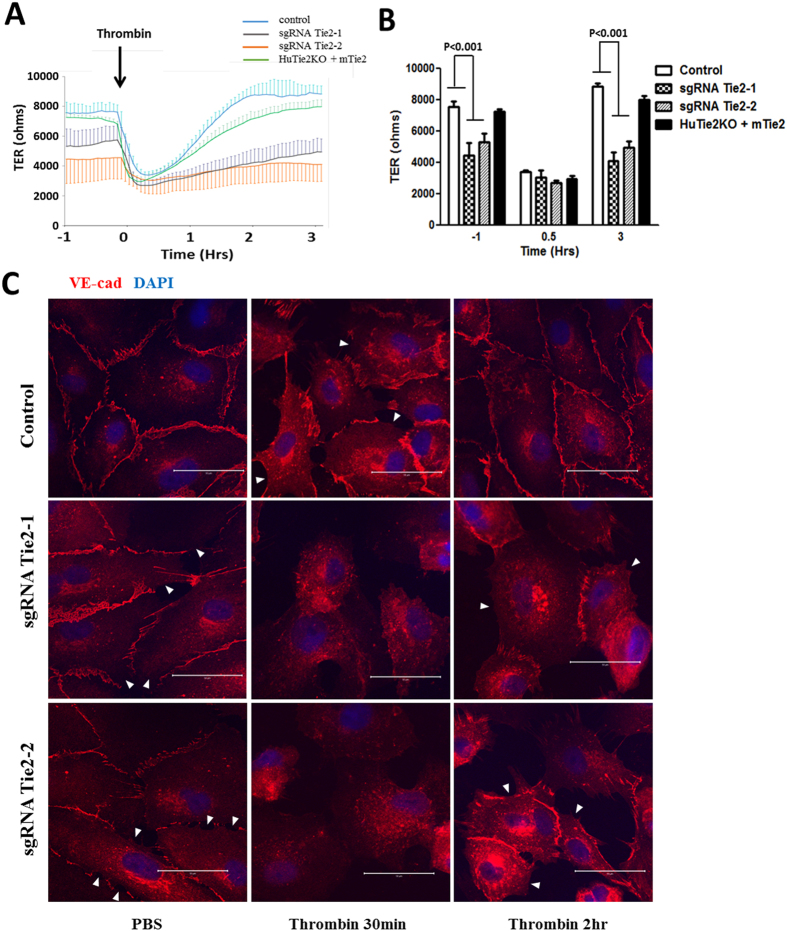
Figure 3. Tie2 deletion by CRISPR-Cas9 in primary ECs increases endothelial permeability and mitigates recovery of permeability in response to thrombin challenge.
(A) Basal TER and post-thrombin (1 U/ml) TER were studied in confluent control, Tie2-deleted HLMVECs and mTie2 overexpressing cells in which Tie2 had been deleted. Absolute TER values were reduced in both Tie2-deleted groups as compared to control ECs at basal condition. mTie2 overexpression successfully rescued the basal leakiness. (B) Quantification of TER values of wild-type (control), transduced cells (sgRNA Tie2-1, sgRNA Tie2-2) and rescued cells (HuTie2KO + mTie2) at basal (−1 h), thrombin-stimulated (0.5 h) and post-recovery (3 h) condition. Differences were calculated using two-way ANOVA. P values less than 0.05 are indicated in the graph. n = 3. (C) Serum-starved confluent control or Tie2-deleted HLMVECs were challenged by PBS or 1 U/ml of thrombin, and subjected for VE-cadherin immunostaining at the indicated time-points and analyzed by confocal microscopy. The marked disruption of VE-cadherin junctions seen in wild-type HLMVEC monolayer (control) at the 30 min post thrombin (white arrows) was reversed by 2 h; however, the defective VE-cadherin junctions were present in Tie2-deleted HLMVECs 2 h post-thrombin. White arrows are used to identify areas of adherens junction disruption where neighboring cells lack cell membrane localization of VE-cadherin. Results are representative of 3 independent experiments.