Abstract
Thermal expansion properties of solids are of fundamental interest and control of thermal expansion is important for practical applications but can be difficult to achieve. Many framework-type materials show negative thermal expansion when internal cages are empty but positive thermal expansion when additional atoms or molecules fill internal voids present. Here we show that redox intercalation offers an effective method to control thermal expansion from positive to zero to negative by insertion of Li ions into the simple negative thermal expansion framework material ScF3, doped with 10% Fe to enable reduction. The small concentration of intercalated Li ions has a strong influence through steric hindrance of transverse fluoride ion vibrations, which directly controls the thermal expansion. Redox intercalation of guest ions is thus likely to be a general and effective method for controlling thermal expansion in the many known framework materials with phonon-driven negative thermal expansion.
The positive thermal expansion exhibited by most materials at increased temperatures is a severe issue for many high precision applications. Here, Xing and co-workers show that redox intercalation of Li ions into a ScF3 framework offers effective control of the thermal expansion for this simple material.
Most materials exhibit positive thermal expansion (PTE) due to the inherent anharmonicity of bond vibrations1,2,3,4 that leads to expansion of average bond distances with increasing temperature. This is a critical issue in many high precision applications subject to large temperature fluctuations such as optical instruments, electronic devices, and spaceflight engineering2,3,4. Thermal expansion control engineering typically makes use of the unconventional property of negative thermal expansion (NTE) which is found in a variety of materials such as oxides1,2,3,4,5,6,7, alloys8,9, nitrides3,10, organic compounds11,12, ReO3-based compounds13,14,15,16,17, metal organic frameworks (MOFs)18,19 and cyanides20. Composites of NTE and PTE materials are often used but may fail after repeated cycling, so direct control of thermal expansion within a single homogenous phase is desirable.
NTE can arise from electronic or magnetic mechanisms, and by a transverse phonon mechanism in insulating framework solids4. These usually have an open structure of corner-sharing metal-anion tetrahedra or octahedra2, for example, the archetype material ZrW2O8 where large NTE was discovered in 1996 (refs 1, 2). NTE has subsequently been reported in many open framework materials such as ReO3-type fluorides21,22,23,24,25, MOFs26,27, cyanides28,29,30, zeolites and AlPO4 frameworks31,32. Although the basic mechanism of NTE in open framework materials through the presence of low energy transverse vibrations is widely accepted, this does not lead to straightforward control of thermal expansion. Chemical substitutions of the framework often have small effects on the lattice dynamics related to expansion, for example, the linear coefficient of thermal expansion (CTE, αl) for Zr1−xMxW2O8−y (M=Sc, In, Y) materials varies only over a short range (−7.3 to −8.7 × 10−6 K−1)33. However, introduction of small molecules such as water into large pore materials is known to have dramatic effects, for example, water adsorption in ZnPt(CN)6·xH2O cyanide34 and ZrW2O8 (ref. 35) switches NTE to PTE behaviour, and thermal expansion is very different between dehydrated36,37 and hydrated forms of cation-exchanged zeolite LTA38.
Hence, a potentially general method for varying thermal expansion is to use intercalation chemistry where small cations such as Li+ can be inserted or removed even from relatively dense frameworks, as much applied in Li-battery chemistry. The intercalated cations are expected to sterically hinder or reduce the transverse vibrations responsible for NTE. We have tested this approach using ScF3 which has a very simple cubic ReO3-type structure, and the results shown here demonstrate that thermal expansion is very effectively tuned from negative to zero or positive values through small changes to the degree of Li ion intercalation.
Results
Composition design for control of thermal expansion in ScF3
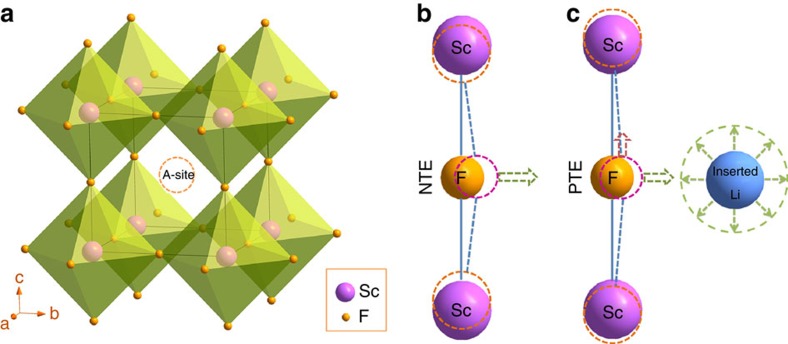
ScF3 has a simple cubic ReO3 crystal structure consisting of a corner-shared ScF6 octahedra (Fig. 1a), equivalent to ABX3 perovskite where the A-site is vacant. ScF3 shows isotropic NTE over a wide range of temperature (10–1,100 K)22,25, and lattice dynamics studies have explored in detail how enhanced transverse thermal vibrations of fluoride ions with increasing temperature lead to shrinkage of Sc–F–Sc linkages and hence NTE25,39 (Fig. 1b). We propose that if those vibrations of fluorine ions can be reduced or hindered by the intercalation of Li ions into the cages of ScF3 (Fig. 1a,c), then control of thermal expansion can be realized.
Figure 1. The effect of Li ion interaction on the tunable thermal expansion of ScF3.
(a) The cubic structure of ScF3 with open framework (space group: 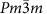 ). The cage consisting of corner-shared ScF3 regular octahedra is marked with the dash line circle (A-site). The guest ions or molecules can be inserted at the A-site cage. (b) The negative thermal expansion of ScF3 induced by the transverse vibration of fluorine normal to the linkage of Sc–F–Sc. (c) The steric hindrance role of inserted ions, eg, Li+, in the vibration of fluorine. The longitudinal vibration of fluorine results in the positive thermal expansion.
). The cage consisting of corner-shared ScF3 regular octahedra is marked with the dash line circle (A-site). The guest ions or molecules can be inserted at the A-site cage. (b) The negative thermal expansion of ScF3 induced by the transverse vibration of fluorine normal to the linkage of Sc–F–Sc. (c) The steric hindrance role of inserted ions, eg, Li+, in the vibration of fluorine. The longitudinal vibration of fluorine results in the positive thermal expansion.
Insertion of Li ions into ScF3 can be achieved by reductive lithiation, which is commonly used for Li-ion battery materials. Sc3+ is not easily reducible, and direct reactions using n-butyllithium failed to form LixScF3 products, so partial substitution of Sc by a reducible metal is needed. The solid solution (Sc0.9Fe0.1)F3 (SFF) was thus synthesized and then lithiated, as described in Methods. The ionic radii of Sc3+, Fe3+ and Fe2+ are, respectively, 0.745, 0.645 and 0.780 Å, so reduction of Fe3+ to Fe2+ in this framework is favoured by lowering of lattice microstrain. Characterization results for SFF and the lithiated product Lix(Sc0.9Fe0.1)F3 are shown in Fig. 2a.
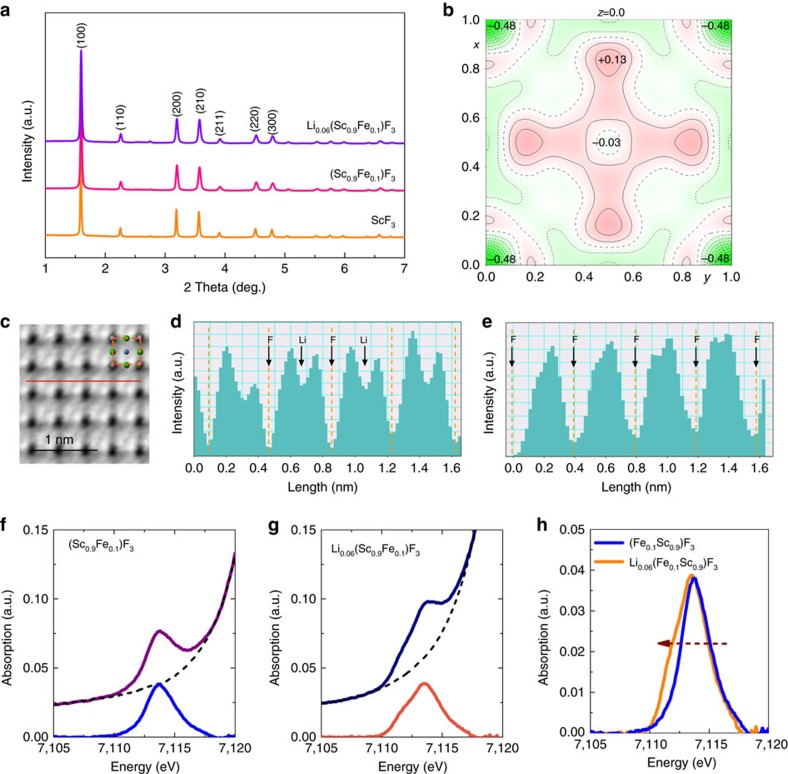
Figure 2. The structure and chemical valence of ScF3-based solid solutions.
(a) High-energy synchrotron X-ray diffraction patterns of ScF3, (Sc0.9Fe0.1)F3 and Li0.06(Sc0.9Fe0.1)F3 samples at room temperature. (b) Difference Fourier map of Li0.06(Sc0.9Fe0.1)F3 at room temperature which was obtained by neutron powder diffraction. The negative intensity indicates that lithium ions are at the A-site. (c) ABF image of lithiated region in the Li0.06(Sc0.9Fe0.1)F3. The inset shows the arrangements of atoms. (d) The corresponding ABF in line profile acquired along the line in c. The black arrows mark the fluorine and lithium atomic sites. (e) The corresponding ABF in line profile in (Sc0.9Fe0.1)F3 without Li as a comparison with d. (f,g) Fe K pre-edge peak extraction for the (Sc0.9Fe0.1)F3 and Li0.06(Sc0.9Fe0.1)F3. (h) The comparison of Fe K pre-edge peak for both (Sc0.9Fe0.1)F3 and Li0.06(Sc0.9Fe0.1)F3 after the background subtraction. The ratio Fe3+/∑Fe can be estimated according to the pre-edge centroid and is reduced in Li0.06(Sc0.9Fe0.1)F3 by lithium intercalation.
Composition determination
The lattice constant increases slightly on Li intercalation, from 3.99368(5) to 3.99927(4) Å for SFF and Lix(Sc0.9Fe0.1)F3, respectively (Fig. 2a), in keeping with lattice expansion due to reduction of Fe3+ to Fe2+. The structure and composition of the lithiated Lix(Sc0.9Fe0.1)F3 product have been determined by joint studies of structure refinement based on neutron powder diffraction (NPD) data, spherical aberration-corrected scanning transmission electron microscopy (STEM) and X-ray absorption near-edge structure (XANES). A neutron scattering Fourier difference map clearly demonstrates that the negative peak at the (0,0,0) is the position of Li ions (Fig. 2b; Supplementary Fig. 1), since Li has a negative neutron scattering length40. Indeed, the refinement is greatly improved by assuming that Li ions are located at the (0,0,0) position in the centre of the perovskite cage (Supplementary Table 1). The chemical composition determined by NPD refinement of the Li occupancy is Li0.06(Sc0.9Fe0.1)F3 (sample LSFF-1; Supplementary Fig. 2), which was further supported by ICP analysis (Supplementary Note 1), so the Li content is below the theoretical maximum of x=0.1. The annular-bright-field (ABF) electron micrographs of LSFF-1 and SFF directly reveal the Li sites within the structural model (Fig. 2c–e), and hence also provide direct evidence for the A-site occupancy of Li ions.
XANES spectra were collected from samples SFF and LSFF-1. There is no change in the Sc K-edge XANES (Supplementary Fig. 3a), which shows that the chemical valence of Sc remains constant during the lithiation. On the other hand, the Fe K-edge is clearly shifted to lower energies, which indicates a partial reduction from Fe3+ to Fe2+ after the lithiation reaction (Supplementary Fig. 3b). The pre-edge peak (Fig. 2f–h), corresponding to the 1s→3d transition, can be used to estimate the Fe3+/∑Fe ratio (Supplementary Note 2). For SFF, the Fe3+/∑Fe ratio is near to unity showing that Fe ions are in the +3 state. However, for LSFF-1, the Fe3+/∑Fe ratio is 0.36, consistent with the 40% residual proportion of Fe3+ ions predicted for the composition Li0.06(Sc0.9Fe0.1)F3 found by NPD refinement.
Tunable thermal expansion via Li intercation
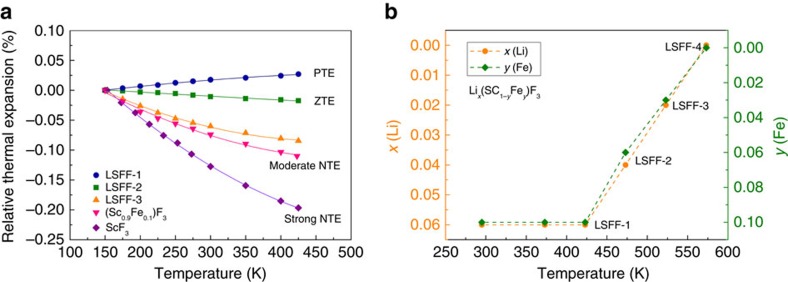
The Lattice parameter measurements demonstrate that Li intercalation has a strong influence on thermal expansion (Fig. 3a). ScF3 and SFF have NTE behaviour with average linear thermal expansions (in the range 150–425 K) of αl=−7.47 and −5.01 × 10−6 K−1, respectively. However, introduction of a small amount of Li at the A-sites results in a change to PTE with αl=1.03 × 10−6 K−1 for LSFF-1 as shown in Fig. 3a. Samples with lower lithium contents were generated by heating LSFF-1 at temperatures above 425 K in inert an N2 atmosphere resulting in loss of Li as LiF and Fe as Fe3O4, and α-Fe2O3 (Supplementary Figs 4–7 and Table 2), and three further Lix(Sc1-yFey)F3 products (samples LSFF-2 to 4) were generated by this route (Fig. 3b). This was used as a convenient way to deintercalate lithium and demonstrate resulting changes in thermal expansion, although it is not a practical method for applications.
Figure 3. The effective control of thermal expansion of ScF3-based compounds.
(a) Temperature evolution of relative change in the lattice constant for the PTE LSFF-1 (Li0.06(Sc0.9Fe0.1)F3 after annealing 425 K), near ZTE LSFF-2 (Li0.04(Sc0.94Fe0.06)F3 after annealing 475 K), moderate NTE LSFF-3 (Li0.02(Sc0.97Fe0.03)F3 after annealing 525 K), moderate NTE (Sc0.9Fe0.1)F3 and strong NTE ScF3. Errors are smaller than the size of data symbols. (b) The stoichiometry of Li and Fe in the main ReO3-type phase, Lix(Sc1-yFey)F3, for the Li0.06(Sc0.9Fe0.1)F3 sample as function of temperature. The stoichiometric values of Li and Fe were estimated according to the content of all phases by means of full-profile Rietveld refinements of neutron powder diffraction (LSFF-1: Li0.06(Sc0.9Fe0.1)F3, LSFF-2: Li0.04(Sc0.94Fe0.06)F3, LSFF-3: Li0.02(Sc0.97Fe0.03)F3, and LSFF-4: ScF3).
Phase contents were determined by structure refinement based on the NPD data, and the results are tabulated in Supplementary Table 3. Their thermal expansion curves in Fig. 3a demonstrate that αl changes smoothly with Li content. Near zero thermal expansion (ZTE) with αl=−0.75 × 10−6 K−1 is achieved in Li0.04(Sc0.94Fe0.06)F3 (LSFF-2) annealed at 475 K, while Li0.02(Sc0.97Fe0.03)F3 (LSFF-3) has moderate NTE of αl=−2.59 × 10−6 K−1. Annealing at 575 K gives sample LSFF-4 with αl=−7.40 × 10−6 K−1 the same as for ScF3 (Supplementary Fig. 8), showing that all Li and Fe have been driven from the framework (Supplementary Note 3). Hence, chemical control of thermal expansion is achieved by adjusting the intercalated Li content. Maximum NTE is obtained for the composition with zero Li content, while weaker NTE, ZTE or PTE can be achieved as Li content increases.
The mechanism of controllable thermal expansion
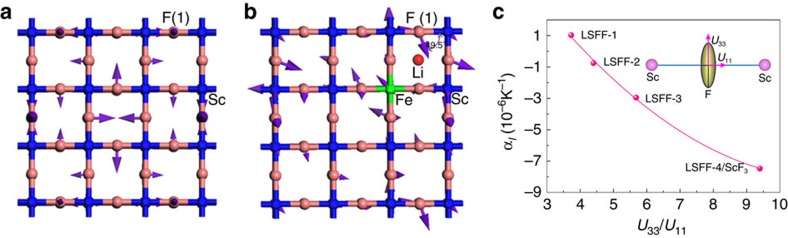
A previous inelastic neutron scattering study revealed that the NTE behaviour of ScF3 mainly originates from the transverse vibration of fluoride ions at low frequencies (0–30 meV)39. To investigate how Li ion intercalation tunes the thermal expansion of ScF3, we have performed vibrational analysis of the fluorine atoms using first-principles calculations. The results reveal that the vibrations of fluorine ions are strongly perturbed by the inserted Li ions, and the directions of all transverse modes are inclined as shown for the representative mode with the lowest frequency in Fig. 4a,b. Figure 4a shows the lowest frequency (37 cm−1) vibrational mode for undoped ScF3 where the vibrational motion of fluorine ions is perpendicular to the Sc–F–Sc linkages leading to NTE in ScF3. Interestingly, intercalation of Li ions into the ScF3 cages strongly perturbs this vibrational mode (Fig. 4b). The vectors of thermal vibration for the closest fluorine ions change from being perpendicular to the Sc–F–Sc linkage to an angle of ∼50° and hence have significant transverse and longitudinal components. This demonstrates that intercalation of Li ions redistributes the fluorine vibrational motion locally, contributing to bond stretching thermal expansion and PTE. Hence even a small concentration of Li (x∼0.04) is sufficient to suppress the overall NTE behaviour, owing to the dual effects on the amplitude and direction of transverse vibrations of fluorine ions.
Figure 4. The role of Li intercalation in control of thermal expansion of ScF3-based solid solutions.
(a) Transverse vibration of fluorine perpendicular to the Sc–F–Sc linkage in the NTE ScF3 super cell. (b) Vibration of fluorine deviating from the perpendicular direction to the Sc–F–Sc linkage in the PTE Li(Sc26Fe)F81 super cell, corresponding to the composition of Li0.037(Sc0.963Fe0.037)F3. Purple arrows indicate the vibration direction of fluorine ions. (c) The correlation between CTE and U33/U11. U33 and U11 are atomic displacement parameters of fluorine ions for the transverse and longitudinal directions, respectively. Larger values of U33/U11 correspond to greater transverse thermal vibration amplitudes of fluorine ions. The inset shows a schematic thermal ellipsoid of fluorine in the Sc–F–Sc linkage.
The effects of Li intercalation on thermal expansion are further supported by the anisotropic atomic amplitude of fluorine ions calculated in the structure refinements from NPD data. Supplementary Table 4 and Fig. 4c show the values of anisotropic atomic displacement parameters of fluorine ions and CTEs of the LSFF compositions. With increasing content of Li the transverse thermal vibration amplitude (U33) of F ions is weakened, while the longitudinal one (U11) is enhanced. As shown in Fig. 4c, there is a good correlation between CTE and the ratio U33/U11. Larger U33/U11 corresponds to stronger transverse thermal vibration of fluorine ions, and a more negative expansion coefficient. The change of thermal expansion from PTE to NTE is accompanied by an increase in the ratio of U33/U11.
Discussion
The above results demonstrate that introducing a small concentration of Li ions into (Sc0.9Fe0.1)F3 switches the thermal expansion behaviour from NTE to PTE, consistent with the Li guest ions in Li0.06(Sc0.9Fe0.1)F3 providing steric hindrance to the transverse vibrations of fluorine ions. Thus, it is likely that NTE can be controlled in many frameworks by adjusting the concentration of guest ions, as previously found for several frameworks by varying the concentration of guest water molecules34,35,36,37,38. This method should be applicable to NTE frameworks containing reducible cations, otherwise substitution can be used to introduce such species such as the small amount of iron replacing scandium in the present example.
The present study may provide an effective method to achieve thermal expansion control engineering by means of the redox intercalation of guest ions or molecules into the pores of a NTE framework. Although we have used chemical intercalation to introduce lithium into (Sc0.9Fe0.1)F3 and thermal decomposition to delithiate leading to impurity formation, standard electrochemical methods used in batteries or sensors could be used to introduce or remove cations reversibly. A similar Li insertion and extraction has been reported to occur reversibly and rapidly at room temperature for ReO3-type FeF3 via an electrochemical method41, validating the feasibility of this approach. Electrochemical methods could lead to smart devices that respond to external stimuli by varying their coefficient of thermal expansion to control complex devices such as precise multicomponent optics. Redox intercalation thus offers a general method for thermal expansion control engineering at the materials and device levels.
In summary, the present study demonstrates that redox intercalation of guest cations into the empty pores of a framework material provides an effective method for tuning thermal expansion properties, in particular in transforming a NTE precursor to a ZTE or PTE product. Intercalated ions play a critical role by sterically hindering the transverse vibrations of corner-shared ScF6 framework polyhedra, and thus changes overall thermal expansion from negative to positive. This method should be applicable to NTE frameworks containing reducible cations or chemical substitution can be used to introduce such species.
Methods
Sample preparation
The (Sc0.9Fe0.1)F3 (SFF) and ScF3 samples were prepared via the solid-state reaction with the precursors of high purity (99.99%) Sc2O3, Fe2O3 and NH4F. These precursors in stoichiometric proportions were pressed into a small pellet (radius and height 5 × 5 mm) and covered with NH4F powder and pressed into a larger pellet (10 × 10 mm). The pellet was loaded into Pt crucible and transferred to a furnace with heating at 600 °C for 5 h, and slow cooling to room temperature. The SFF sample was found to be phase pure by X-ray diffraction.
The Li0.06(Sc0.9Fe0.1)F3 sample was obtained by the chemical intercalation of the SFF powder with n-butyllithium (1.6 M in hexane Aldrich, approximately 10 times the stoichiometric mole ratio) at room temperature for 24 h in a glove box with high purity argon atmosphere. The as-prepared sample powder was washed with hexane several times, and then dried under N2 flow at 80 °C for 10 h. The product did not decompose on heating up to 425 K giving composition Li0.06(Sc0.9Fe0.1)F3 (sample LSFF-1). Samples Li0.04(Sc0.94Fe0.06)F3 (LSFF-2), Li0.02(Sc0.97Fe0.03)F3 (LSFF-3) and ScF3 (LSFF-4) were obtained by partial thermal decomposition in an inert N2 atmosphere, respectively, at 475, 525 and 575 K.
A small amount (0.8 wt%) of impurity phase LiF was observed in LSFF-1. Further impurity phases of iron oxides in LSFF-2 to 4 were generated by high-temperature decomposition as shown in Supplementary Table 3. It needs to note that the presence of impurity phases does not affect the intrinsic thermal expansion properties of the ReO3-type phases.
X-ray and neutron powder diffraction
Temperature dependent X-ray diffraction data were collected from 150 to 650 K by using a PANalytical, PW 3040-X-PertPro X-ray diffractometer. The lattice constant was refined using a cubic structural model (space group: 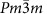 ). Room temperature synchrotron X-ray diffraction data were collected at the instrument 11-ID-C at the Advanced Photon Source with a wavelength of 0.111650 Å. NPD data of the Li0.06(Sc0.9Fe0.1)F3 sample was collected from 298 to 573 K at the NIST Center for Neutron Research on the BT-1 high-resolution neutron powder diffractometer. The wavelength of the neutron beam was 1.5398 Å. The chemical compositions and anisotropic atomic displacement parameters of fluorine ions of the LSFF samples were determined by structure refinement using NPD data. All structure calculations were performed using FULLPROF software42. The details of refinements can be found in Supplementary Note 1.
). Room temperature synchrotron X-ray diffraction data were collected at the instrument 11-ID-C at the Advanced Photon Source with a wavelength of 0.111650 Å. NPD data of the Li0.06(Sc0.9Fe0.1)F3 sample was collected from 298 to 573 K at the NIST Center for Neutron Research on the BT-1 high-resolution neutron powder diffractometer. The wavelength of the neutron beam was 1.5398 Å. The chemical compositions and anisotropic atomic displacement parameters of fluorine ions of the LSFF samples were determined by structure refinement using NPD data. All structure calculations were performed using FULLPROF software42. The details of refinements can be found in Supplementary Note 1.
X-ray absorption fine structure spectroscopy
Sc and Fe K-edge XANES and extended X-ray absorption fine structure (EXAFS) spectroscopy measurements were performed from room temperature to about 700 K at the XAFS beamline of ELETTRA synchrotron radiation facility in Trieste (Italy). The samples for EXAFS were prepared by mixing and pelletizing the SFF and Li0.06(Sc0.9Fe0.1)F3 powder with boron nitride powder. The EXAFS spectra were collected in transmission mode in the energy range 4.3–5.7 keV for Sc K-edge EXAFS, 6.9–8.3 keV for Fe K-edge EXAFS, with an energy step varying from 0.1 eV in the near-edge region to about 4 eV at the highest energies, to obtain a uniform wave vector step Δk ≅ 0.03 Å−1. The X-ray beam was monochromatized by a Si(111) double-crystal monochromator. The sample was mounted in a high-temperature furnace and the temperature was stabilized and monitored through an electric heater controlled by a feedback loop, ensuring a thermal stability within ±1 K.
Scanning transmission electron microscopy
STEM was performed using a JEM-ARM 200F (JEOL, Tokyo, Japan) that operated at 200 kV and was equipped with double aberration-correctors for probe-forming. Imaging lenses were used to perform high-angle annular-dark field (HAADF) and ABF imaging. The attainable spatial resolution of the microscope is 78 pm at the incident semi-angle of 25 mrad. To observe Li ions directly using ABF collection geometry, the acceptance semi-angle in this study was fixed between 12 and 25 mrad.
First-principles calculation
The first-principles vibrational analysis was performed by CASTEP43, a total energy package based on the plane-wave pseudopotential density functional theory method44,45. The exchange-correlation functional developed by Perdew, Burke and Ernzerhof46 in general gradient approximation form ref. 47 was adopted to describe the exchange-correlation energy. The effective interaction between the valence electrons (Li 2s1, Sc 3d14s2, Fe 3d64s2 and F 2s22p5) and atom cores were modelled by optimized norm-conversing pseudopotentials48, which allow us to choose a relatively small plane wave basis set without compromising the computational accuracy. The kinetic energy cutoff 900 eV and dense Monkhorst-Pack49 k-point mesh spanning less than 0.04 Å−1 in the Brillouin zone were chosen. To consider the effect of the intercalation of Li ions on the vibrational property, a 3 × 3 × 3 super cell was built in which one Sc atom was replaced by Fe atom and one Li atom was inserted in the neighbouring A-site. Before vibrational property calculation, the crystal structure was geometrically optimized to find the energy minimum. The Broyden–Fletcher–Goldfarb–Shanno (BFGS) minimization scheme50 was employed in the geometry optimization, in which the convergence criteria for the structure optimization were set to 5.0 × 10−5 eV per atom, 0.1 eV Å−1, 0.2 GPa and 5.0 × 10−3 GPa for energy, maximum force, maximum stress and maximum displacement, respectively. On the basis of crystal configuration in the minimal energy, the frequencies of the phonon modes were calculated by linear response formalism51, and the phonon modes were obtained by the second derivative of the total energy with respect to a given perturbation.
Data availability
The data relevant to the findings of this study are available from the corresponding authors on reasonable request.
Additional information
How to cite this article: Chen, J. et al. Tunable thermal expansion in framework materials through redox intercalation. Nat. Commun. 8, 14441 doi: 10.1038/ncomms14441 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures, Supplementary Tables, Supplementary Notes and Supplementary References
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant nos. 21322102, 91422301, 21231001, 21590793 and 11474292), the Program for Changjiang Scholars and the Innovative Research Team in University (IRT1207), the Changjiang Young Scholars Award, National Program for Support of Top-notch Young Professionals, and the Fundamental Research Funds for the Central Universities, China (FRF-TP-14-012C1), the Special Foundation of the Director of Technical Institute of Physics and Chemistry (TIPC). The use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences (DE-AC02-06CH11357). We acknowledge the ELETTRA Synchrotron Radiation Facility for provision of synchrotron radiation as well as all the staff of the XAFS beamline.
Footnotes
The authors declare no competing financial interests.
Author contributions J.C., J.P.A. and X.X. initiated and designed the research. J.C., J.P.A. and X.X. discussed and wrote the manuscript, and all authors revised and commented on it. J.C., Q.G., K.L. and Q.H. measured and analysed the neutron diffraction data. Q.G. synthesized the samples. Q.G. and J.D. measured and analysed the thermal expansion data. A.S., A.C., C.G.R. and L.O. collected the data of EXAFS and A.S. analysed the data of EXAFS. L.G. measured and helped to analyse the STEM data. X.J., Z.L., L.W. and C.W. performed the first-principle calculations, and X.J. wrote the content of theoretical calculations. Y.R. and L.H. measured and helped to analyse the data of synchrotron X-ray diffraction. J.C. and X.X. guided the projects.
References
- Mary T. A., Evans J. S. O., Vogt T. & Sleight A. W. Negative thermal expansion from 0.3 to 1,050 Kelvin in ZrW2O8. Science 272, 90–92 (1996). [Google Scholar]
- Evans J. S. O. Negative thermal expansion materials. J. Chem. Soc. Dalton. Trans. 19, 3317–3326 (1999). [Google Scholar]
- Takenaka K. Negative thermal expansion materials: technological key for control of thermal expansion. Sci. Technol. Adv. Mater. 13, 013001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Hu L., Deng J. X. & Xing X. R. Negative thermal expansion in functional materials: controllable thermal expansion by chemical modifications. Chem. Soc. Rev. 44, 3522–3567 (2015). [DOI] [PubMed] [Google Scholar]
- Chen J. et al. Zero thermal expansion in PbTiO3-based perovskites. J. Am. Chem. Soc. 130, 1144–1145 (2008). [DOI] [PubMed] [Google Scholar]
- Azuma M. et al. Colossal negative thermal expansion in BiNiO3 induced by intermetallic charge transfer. Nat. Commun. 2, 347 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn M. S. et al. Symmetry switching of negative thermal expansion by chemical control. J. Am. Chem. Soc. 138, 5479–5482 (2016). [DOI] [PubMed] [Google Scholar]
- Mohn P. Materials science: a century of zero expansion. Nature 400, 18–19 (1999). [Google Scholar]
- Huang R. J. et al. Giant negative thermal expansion in NaZn13-type La(Fe,Si,Co)13 compounds. J. Am. Chem. Soc. 135, 11469–11472 (2013). [DOI] [PubMed] [Google Scholar]
- Takenaka K. & Takagi H. Giant negative thermal expansion in Ge-doped anti-perovskite manganese nitrides. Appl. Phys. Lett. 87, 1902 (2005). [Google Scholar]
- Das D., Jacobs T. & Barbour L. J. Exceptionally large positive and negative anisotropic thermal expansion of an organic crystalline material. Nat. Mater. 9, 36–39 (2010). [DOI] [PubMed] [Google Scholar]
- Birkedal H., Schwarzenbach D. & Pattison P. Observation of uniaxial negative thermal expansion in an organic crystal. Angew. Chem. Int. Ed. 114, 780–782 (2002). [DOI] [PubMed] [Google Scholar]
- Morelock C. R., Greve B. K., Gallington L. C., Chapman K. W. & Wilkinson A. P. Negative thermal expansion and compressibility of Sc1–xYxF3 (x≤0.25). J. Appl. Phys. 114, 213501 (2013). [Google Scholar]
- Wilkinson A. P., Josefsberg R. E., Gallington L. C., Morelock C. R. & Monaco C. M. History-dependent thermal expansion in NbO2F. J. Solid State Chem. 213, 38–42 (2014). [Google Scholar]
- Han F. et al. The distortion-adjusted change of thermal expansion behavior of cubic magnetic semiconductor (Sc1−xMx)F3 (M=Al, Fe). J. Am. Ceram. Soc. 99, 2886–2888 (2016). [Google Scholar]
- Hu L. et al. Zero thermal expansion and ferromagnetism in cubic Sc1−xMxF3 (M=Ga, Fe) over a wide temperature range. J. Am. Chem. Soc. 136, 13566–13569 (2014). [DOI] [PubMed] [Google Scholar]
- Morelock C. R., Gallington L. C. & Wilkinson A. P. Solid solubility, phase transitions, thermal expansion, and compressibility in Sc1−xAlxF3. J. Solid State Chem. 222, 96–102 (2015). [Google Scholar]
- Lama P., Das R. K., Smith V. J. & Barbour L. J. A combined stretching–tilting mechanism produces negative, zero and positive linear thermal expansion in a semi-flexible Cd (ii)-MOF. Chem. Commun. 50, 6464–6467 (2014). [DOI] [PubMed] [Google Scholar]
- Grobler I., Smith V. J., Bhatt P. M., Herbert S. A. & Barbour L. J. Tunable anisotropic thermal expansion of a porous zinc (II) metal–organic framework. J. Am. Chem. Soc. 135, 6411–6414 (2013). [DOI] [PubMed] [Google Scholar]
- Duyker S. G., Peterson V. K., Kearley G. J., Ramirez-Cuesta A. J. & Kepert C. J. Negative thermal expansion in LnCo(CN)6 (Ln= La, Pr, Sm, Ho, Lu, Y): mechanisms and compositional trends. Angew. Chem. Int. Ed. 52, 5266–5270 (2013). [DOI] [PubMed] [Google Scholar]
- Attfield J. P. Condensed-matter physics: a fresh twist on shrinking materials. Nature 480, 465–466 (2011). [DOI] [PubMed] [Google Scholar]
- Greve B. K. et al. Pronounced negative thermal expansion from a simple structure: Cubic ScF3. J. Am. Chem. Soc. 132, 15496–15498 (2010). [DOI] [PubMed] [Google Scholar]
- Hancock J. C. et al. Large negative thermal expansion and anomalous behavior on compression in cubic ReO3-type AIIBIVF6: CaZrF6 and CaHfF6. Chem. Mater. 27, 3912–3918 (2015). [Google Scholar]
- Romao C. P. et al. The heat capacities of thermomiotic ScF3 and ScF3-YF3 solid solutions. J. Mater. Sci. 50, 3409–3415 (2015). [Google Scholar]
- Hu L. et al. New insights into the negative thermal expansion: direct experimental evidence for the ‘guitar-string' effect in cubic ScF3. J. Am. Chem. Soc. 138, 8320–8323 (2016). [DOI] [PubMed] [Google Scholar]
- Dubbeldam D. et al. Exceptional negative thermal expansion in isoreticular metal–organic frameworks. Angew. Chem. Int. Ed. 119, 4580–4583 (2007). [DOI] [PubMed] [Google Scholar]
- Zhou H.-L. et al. Direct visualization of a guest-triggered crystal deformation based on a flexible ultramicroporous framework. Nat. Commun. 4, 2534 (2013). [DOI] [PubMed] [Google Scholar]
- Margadonna S., Prassides K. & Fitch A. N. Zero thermal expansion in a Prussian blue analogue. J. Am. Chem. Soc. 126, 15390–15391 (2004). [DOI] [PubMed] [Google Scholar]
- Chapman K. W., Chupas P. J. & Kepert C. J. Compositional dependence of negative thermal expansion in the Prussian blue analogues MIIPtIV (CN)6 (M=Mn, Fe, Co, Ni, Cu, Zn, Cd). J. Am. Chem. Soc. 128, 7009–7014 (2006). [DOI] [PubMed] [Google Scholar]
- Goodwin A. L. et al. Colossal positive and negative thermal expansion in the framework material Ag3[Co(CN)6]. Science 319, 794–797 (2008). [DOI] [PubMed] [Google Scholar]
- Cha W. et al. Core–shell strain structure of zeolite microcrystals. Nat. Mater. 12, 729–734 (2013). [DOI] [PubMed] [Google Scholar]
- Lightfoot P., Woodcock D. A., Maple M. J., Villaescusa L. A. & Wright P. A. The widespread occurrence of negative thermal expansion in zeolites. J. Mater. Chem. 11, 212–216 (2001). [Google Scholar]
- Nakajima N., Yamamura Y. & Tsuji T. Synthesis and physical properties of negative thermal expansion materials Zr1−xMxW2O8−y (M=Sc, In, Y) substituted for Zr (IV) sites by M (III) ions. Solid State Commun. 128, 193–196 (2003). [Google Scholar]
- Goodwin A. L., Chapman K. W. & Kepert C. J. Guest-dependent negative thermal expansion in nanoporous prussian blue analogues MIIPtIV(CN)6 x{H2O}(0≤x≤2; M=Zn, Cd). J. Am. Chem. Soc. 127, 17980–17981 (2015). [DOI] [PubMed] [Google Scholar]
- Duan N., Kameswari U. & Sleight A. W. Further contraction of ZrW2O8. J. Am. Chem. Soc. 121, 10432–10433 (1999). [Google Scholar]
- Phillips A. E., Goodwin A. L., Halder G. J., Southon P. D. & Kepert C. J. Nanoporosity and exceptional negative thermal expansion in single-network cadmium cyanide. Angew. Chem. Int. Ed. 120, 1418–1421 (2008). [DOI] [PubMed] [Google Scholar]
- Phillips A. E., Halder G. J., Chapman K. W., Goodwin A. L. & Kepert C. J. Zero thermal expansion in a flexible, stable framework: tetramethylammonium copper (I) zinc (II) cyanide. J. Am. Chem. Soc. 132, 10–11 (2009). [DOI] [PubMed] [Google Scholar]
- Carey T., Tang C. C., Hriljac J. A. & Anderson P. A. Chemical control of thermal expansion in cation-exchanged zeolite A. Chem. Mater. 26, 1561–1566 (2014). [Google Scholar]
- Li C. W. et al. Structural relationship between negative thermal expansion and quartic anharmonicity of cubic ScF3. Phys. Rev. Lett. 107, 195504 (2011). [DOI] [PubMed] [Google Scholar]
- Pérez-Estébanez M., Isasi-Marín J., Többens D. M., Rivera-Calzada A. & León C. A systematic study of Nasicon-type Li1+xMxTi2−x(PO4)3 (M: Cr, Al, Fe) by neutron diffraction and impedance spectroscopy. Solid State Ionics 266, 1–8 (2014). [Google Scholar]
- Liu P. et al. Thermodynamics and kinetics of the Li/FeF3 reaction by electrochemical analysis. J. Phys. Chem. C. 116, 6467–6473 (2012). [Google Scholar]
- Rodríguez-Carvajal J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 192, 55–69 (1993). [Google Scholar]
- Clark S. J. et al. First principles methods using CASTEP. Z. Kristallogr. 220, 567–570 (2005). [Google Scholar]
- Kohn W. & Sham L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965). [Google Scholar]
- Payne M. C., Teter M. P., Allan D. C., Arias T. A. & Joannopoulos J. D. Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 64, 1045–1097 (1992). [Google Scholar]
- Perdew J. P., Burke K. & Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
- Perdew J. P. et al. Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B. 46, 6671–6687 (1992). [DOI] [PubMed] [Google Scholar]
- Rappe A. M., Rabe K. M., Kaxiras E. & Joannopoulos J. D. Optimized pseudopotentials. Phys. Rev. B. 41, 1227–1230 (1990). [DOI] [PubMed] [Google Scholar]
- Monkhorst H. J. & Pack J. P. Special points for brillouin-zone integrations. Phys. Rev. B. 13, 5188–5192 (1976). [Google Scholar]
- Pfrommer B. G., Côté M., Louie S. G. & Cohen M. L. Relaxation of crystals with the quasi-newton method. J. Comput. Phys. 131, 233–240 (1997). [Google Scholar]
- Baroni S., De Gironcoli S., Dal Corso A. & Giannozzi P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 73, 515–562 (2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures, Supplementary Tables, Supplementary Notes and Supplementary References
Data Availability Statement
The data relevant to the findings of this study are available from the corresponding authors on reasonable request.